当前位置:
X-MOL 学术
›
ACS Med. Chem. Lett.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Multiple in Vitro Inhibition of HIV-1 Proteins by 2,6-Dipeptidyl-anthraquinone Conjugates Targeting the PBS RNA.
ACS Medicinal Chemistry Letters ( IF 4.2 ) Pub Date : 2020-03-23 , DOI: 10.1021/acsmedchemlett.9b00682 Elia Gamba 1 , Alice Sosic 1 , Irene Saccone 2 , Elisa Magli 2 , Francesco Frecentese 2 , Barbara Gatto 1
ACS Medicinal Chemistry Letters ( IF 4.2 ) Pub Date : 2020-03-23 , DOI: 10.1021/acsmedchemlett.9b00682 Elia Gamba 1 , Alice Sosic 1 , Irene Saccone 2 , Elisa Magli 2 , Francesco Frecentese 2 , Barbara Gatto 1
Affiliation
|
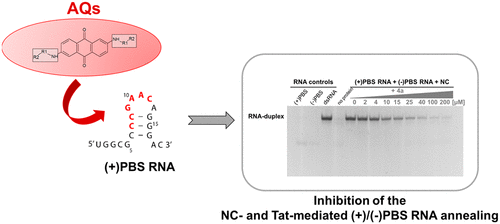
We recently reported a series of 2,6-dipeptidyl-anthraquinone conjugates (AQs) as Trans-Activation Response element (TAR) RNA-binding agents able to inhibit in vitro the HIV-1 nucleocapsid (NC) protein-mediated processes. Because NC is a highly adaptable nucleic acid chaperone assisting several crucial steps along reverse transcription, in this study we investigate the ability of AQs to interact with other virus-derived nucleic acid structures thus potentially inhibiting multiple NC functions. Focusing on the HIV-1 Primer Binding Site (PBS) RNA sequence, we demonstrate that properly substituted dipeptidyl-anthraquinone conjugates efficiently inhibit the NC-mediated primer annealing in the low micromolar range. Similarly, we extended the analysis to the HIV-1 trans-activator of transcription (Tat) peptide, which has been recently shown to mimic the annealer functions of NC upon interacting with the same nucleic acid regulatory sequences. Our results highlight how RNA-targeting agents can act as multimode inhibitors of key viral proteins affecting their chaperone activity in reverse transcription processes.
中文翻译:

2,6-二肽基-蒽醌对HIV-1蛋白的多重体外抑制作用结合了靶向PBS RNA。
我们最近报道了一系列的2,6-二肽基-蒽醌共轭物(AQs)作为反式激活元件(TAR)RNA结合剂,能够在体外抑制HIV-1核衣壳(NC)蛋白介导的过程。由于NC是一种高度适应性的核酸分子伴侣,可协助逆转录过程中的几个关键步骤,因此在本研究中,我们研究了AQ与其他病毒衍生核酸结构相互作用的能力,从而潜在地抑制了多种NC功能。专注于HIV-1引物结合位点(PBS)RNA序列,我们证明适当取代的二肽基-蒽醌共轭物可在低微摩尔范围内有效抑制NC介导的引物退火。同样,我们将分析扩展到了HIV-1转录反式激活子(Tat)肽,最近显示,其在与相同的核酸调节序列相互作用时模仿NC的退火功能。我们的结果突出了RNA靶向剂如何充当关键病毒蛋白的多模抑制剂,从而影响其在逆转录过程中的伴侣活性。
更新日期:2020-03-23
中文翻译:

2,6-二肽基-蒽醌对HIV-1蛋白的多重体外抑制作用结合了靶向PBS RNA。
我们最近报道了一系列的2,6-二肽基-蒽醌共轭物(AQs)作为反式激活元件(TAR)RNA结合剂,能够在体外抑制HIV-1核衣壳(NC)蛋白介导的过程。由于NC是一种高度适应性的核酸分子伴侣,可协助逆转录过程中的几个关键步骤,因此在本研究中,我们研究了AQ与其他病毒衍生核酸结构相互作用的能力,从而潜在地抑制了多种NC功能。专注于HIV-1引物结合位点(PBS)RNA序列,我们证明适当取代的二肽基-蒽醌共轭物可在低微摩尔范围内有效抑制NC介导的引物退火。同样,我们将分析扩展到了HIV-1转录反式激活子(Tat)肽,最近显示,其在与相同的核酸调节序列相互作用时模仿NC的退火功能。我们的结果突出了RNA靶向剂如何充当关键病毒蛋白的多模抑制剂,从而影响其在逆转录过程中的伴侣活性。



























 京公网安备 11010802027423号
京公网安备 11010802027423号