当前位置:
X-MOL 学术
›
ACS Chem. Neurosci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Investigation of the 2,5-Dimethoxy Motif in Phenethylamine Serotonin 2A Receptor Agonists.
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2020-04-13 , DOI: 10.1021/acschemneuro.0c00129 Emil Marcher-Rørsted 1 , Adam L Halberstadt 2 , Adam K Klein 2 , Muhammad Chatha 2 , Simon Jademyr 1 , Anders A Jensen 1 , Jesper L Kristensen 1
ACS Chemical Neuroscience ( IF 5 ) Pub Date : 2020-04-13 , DOI: 10.1021/acschemneuro.0c00129 Emil Marcher-Rørsted 1 , Adam L Halberstadt 2 , Adam K Klein 2 , Muhammad Chatha 2 , Simon Jademyr 1 , Anders A Jensen 1 , Jesper L Kristensen 1
Affiliation
|
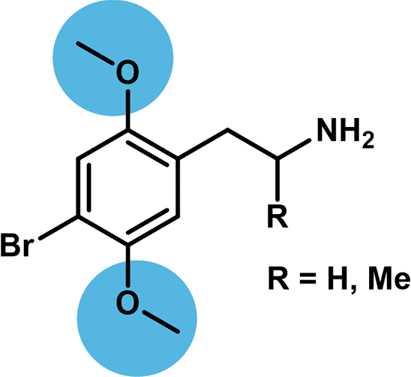
The 2,5-dimethoxyphenethylamine (2,5-PEA) scaffold is recognized as a motif conferring potent agonist activity at the serotonin 2A receptor (5-HT2AR). The 2,5-dimethoxy motif is present in several classical phenethylamine psychedelics such as 2,4,5- trimethoxyamphetamine (TMA-2), 2,5-dimethoxy-4-methylamphetamine (DOM), 2,5-dimethoxy-4-iodoamphetamine (DOI), 2,5- dimethoxy-4-bromoamphetamine (DOB), 2,5-dimethoxy-4-bromophenethylamine (2C-B), and 2,5-dimethoxy-4-iodophenethylamine (2C-I), and it has previously been suggested that this structural motif is essential for 5-HT2AR activation. In the present study, we present data that challenges this assumption. The 2- and 5-desmethoxy derivatives of 2C-B and DOB were synthesized, and their pharmacological profiles were evaluated in vitro at 5-HT2AR and 5-HT2CR in binding and functional assays and in vivo by assessing their induction of the head-twitch response in mice. Elimination of either the 2- or 5-methoxy group leads to a modest drop in binding affinity and functional potency at 5-HT2AR and 5-HT2CR, which was more pronounced upon removal of the 2-methoxy group. However, this trend was not mirrored in vivo, as removal of either methoxy group resulted in significant reduction in the ability of the compounds to induce the head-twitch response in mice. Thus, the 2,5-dimethoxyphenethylamine motif appears to be important for in vivo potency of phenethylamine 5-HT2AR agonists, but this does not correlate to the relative affinity and potency of the ligands at the recombinant 5-HT2AR.
中文翻译:

苯乙胺血清素2A受体激动剂中的2,5-二甲氧基基序的研究。
2,5-二甲氧基苯乙胺(2,5-PEA)支架被认为是赋予5-羟色胺2A受体(5-HT2AR)潜在激动剂活性的基序。2,5-二甲氧基基序存在于几种经典的苯乙胺迷幻药中,例如2,4,5-三甲氧基苯丙胺(TMA-2),2,5-二甲氧基-4-甲基苯丙胺(DOM),2,5-二甲氧基-4-碘苯丙胺(DOI),2,5-二甲氧基-4-溴苯丙胺(DOB),2,5-二甲氧基-4-溴苯乙胺(2C-B)和2,5-二甲氧基-4-碘苯乙胺(2C-1)和先前已经提出,该结构基序对于5-HT 2AR活化是必不可少的。在本研究中,我们提出了挑战这一假设的数据。合成了2C-B和DOB的2-和5-去甲氧基衍生物 通过在结合和功能测定中在5-HT2AR和5-HT2CR的体外评估其药理学特征,并通过评估它们对小鼠头抽搐反应的诱导来评估其药理学特征。消除2-或5-甲氧基基团导致在5-HT 2AR和5-HT 2CR的结合亲和力和功能效力适度下降,这在去除2-甲氧基基团时更为明显。但是,这种趋势在体内并未得到反映,因为任何一个甲氧基的去除都会导致化合物诱导小鼠头跳反应的能力显着降低。因此,2,5-二甲氧基苯乙胺基序对于苯乙胺5-HT 2AR激动剂的体内效力似乎很重要,但这与配体在重组5-HT 2AR上的相对亲和力和效力无关。
更新日期:2020-03-26
中文翻译:

苯乙胺血清素2A受体激动剂中的2,5-二甲氧基基序的研究。
2,5-二甲氧基苯乙胺(2,5-PEA)支架被认为是赋予5-羟色胺2A受体(5-HT2AR)潜在激动剂活性的基序。2,5-二甲氧基基序存在于几种经典的苯乙胺迷幻药中,例如2,4,5-三甲氧基苯丙胺(TMA-2),2,5-二甲氧基-4-甲基苯丙胺(DOM),2,5-二甲氧基-4-碘苯丙胺(DOI),2,5-二甲氧基-4-溴苯丙胺(DOB),2,5-二甲氧基-4-溴苯乙胺(2C-B)和2,5-二甲氧基-4-碘苯乙胺(2C-1)和先前已经提出,该结构基序对于5-HT 2AR活化是必不可少的。在本研究中,我们提出了挑战这一假设的数据。合成了2C-B和DOB的2-和5-去甲氧基衍生物 通过在结合和功能测定中在5-HT2AR和5-HT2CR的体外评估其药理学特征,并通过评估它们对小鼠头抽搐反应的诱导来评估其药理学特征。消除2-或5-甲氧基基团导致在5-HT 2AR和5-HT 2CR的结合亲和力和功能效力适度下降,这在去除2-甲氧基基团时更为明显。但是,这种趋势在体内并未得到反映,因为任何一个甲氧基的去除都会导致化合物诱导小鼠头跳反应的能力显着降低。因此,2,5-二甲氧基苯乙胺基序对于苯乙胺5-HT 2AR激动剂的体内效力似乎很重要,但这与配体在重组5-HT 2AR上的相对亲和力和效力无关。



























 京公网安备 11010802027423号
京公网安备 11010802027423号