当前位置:
X-MOL 学术
›
Anal. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Evaluation of Sibling and Twin Fragment Ions Improves the Structural Characterization of Proteins by Top-Down MALDI In-Source Decay Mass Spectrometry.
Analytical Chemistry ( IF 6.7 ) Pub Date : 2020-04-02 , DOI: 10.1021/acs.analchem.9b05683 Simone Nicolardi 1 , David P A Kilgour 2 , Natasja Dolezal 3 , Jan W Drijfhout 3 , Manfred Wuhrer 1 , Yuri E M van der Burgt 1
Analytical Chemistry ( IF 6.7 ) Pub Date : 2020-04-02 , DOI: 10.1021/acs.analchem.9b05683 Simone Nicolardi 1 , David P A Kilgour 2 , Natasja Dolezal 3 , Jan W Drijfhout 3 , Manfred Wuhrer 1 , Yuri E M van der Burgt 1
Affiliation
|
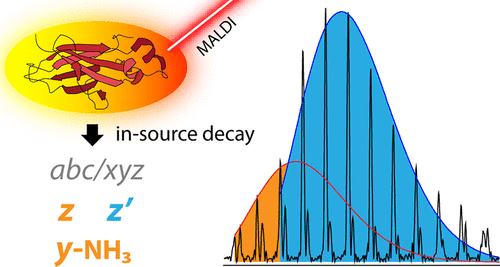
Comprehensive determination of primary sequence and identification of post-translational modifications (PTMs) are key elements in protein structural analysis. Various mass spectrometry (MS) based fragmentation techniques are powerful approaches for mapping both the amino acid sequence and PTMs; one of these techniques is matrix-assisted laser desorption/ionization (MALDI), combined with in-source decay (ISD) fragmentation and Fourier-transform ion cyclotron resonance (FT-ICR) MS. MALDI-ISD MS protein analysis involves only minimal sample preparation and does not require spectral deconvolution. The resulting MALDI-ISD MS data is complementary to electrospray ionization-based MS/MS sequencing readouts, providing knowledge on the types of fragment ions is available. In this study, we evaluate the isotopic distributions of z' ions in protein top-down MALDI-ISD FT-ICR mass spectra and show why these distributions can deviate from theoretical profiles as a result of co-occurring and isomeric z and y-NH3 ions. Two synthetic peptides, containing either normal or deuterated alanine residues, were used to confirm the presence and unravel the identity of isomeric z and y-NH3 fragment ions ("twins"). Furthermore, two reducing MALDI matrices, namely 1,5-diaminonaphthalene and N-phenyl-p-phenylenediamine were applied that yield ISD mass spectra with different fragment ion distributions. This study demonstrates that the relative abundance of isomeric z and y-NH3 ions requires consideration for accurate and confident assignments of z' ions in MALDI-ISD FT-ICR mass spectra.
中文翻译:

通过自上而下的MALDI源内衰减质谱分析法评估同级和双碎片离子可改善蛋白质的结构表征。
初级序列的全面确定和翻译后修饰(PTM)的识别是蛋白质结构分析中的关键要素。各种基于质谱(MS)的片段化技术都是用于定位氨基酸序列和PTM的强大方法。这些技术之一是基质辅助激光解吸/电离(MALDI),源内衰减(ISD)碎裂和傅立叶变换离子回旋共振(FT-ICR)MS。MALDI-ISD MS蛋白质分析仅涉及最少的样品制备,并且不需要光谱解卷积。所得的MALDI-ISD MS数据可补充基于电喷雾电离的MS / MS序列读数,并提供有关碎片离子类型的知识。在这项研究中,我们评估z'的同位素分布 自上而下的MALDI-ISD FT-ICR质谱图中的氢离子,并显示了为什么由于共生和异构的z和y-NH3离子,这些分布可能偏离理论曲线。含有正常或氘代丙氨酸残基的两种合成肽被用于确认存在和解开同分异构的z和y-NH 3片段离子的身份(“双胞胎”)。此外,应用了两种还原性MALDI基质,即1,5-二氨基萘和N-苯基-对苯二胺,可产生具有不同碎片离子分布的ISD质谱图。这项研究表明,z和y-NH3异构体离子的相对丰度需要考虑MALDI-ISD FT-ICR质谱图中z'离子的准确和可靠分配。
更新日期:2020-04-23
中文翻译:

通过自上而下的MALDI源内衰减质谱分析法评估同级和双碎片离子可改善蛋白质的结构表征。
初级序列的全面确定和翻译后修饰(PTM)的识别是蛋白质结构分析中的关键要素。各种基于质谱(MS)的片段化技术都是用于定位氨基酸序列和PTM的强大方法。这些技术之一是基质辅助激光解吸/电离(MALDI),源内衰减(ISD)碎裂和傅立叶变换离子回旋共振(FT-ICR)MS。MALDI-ISD MS蛋白质分析仅涉及最少的样品制备,并且不需要光谱解卷积。所得的MALDI-ISD MS数据可补充基于电喷雾电离的MS / MS序列读数,并提供有关碎片离子类型的知识。在这项研究中,我们评估z'的同位素分布 自上而下的MALDI-ISD FT-ICR质谱图中的氢离子,并显示了为什么由于共生和异构的z和y-NH3离子,这些分布可能偏离理论曲线。含有正常或氘代丙氨酸残基的两种合成肽被用于确认存在和解开同分异构的z和y-NH 3片段离子的身份(“双胞胎”)。此外,应用了两种还原性MALDI基质,即1,5-二氨基萘和N-苯基-对苯二胺,可产生具有不同碎片离子分布的ISD质谱图。这项研究表明,z和y-NH3异构体离子的相对丰度需要考虑MALDI-ISD FT-ICR质谱图中z'离子的准确和可靠分配。











































 京公网安备 11010802027423号
京公网安备 11010802027423号