当前位置:
X-MOL 学术
›
ACS Appl. Nano Mater.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Mesoporous Copper Nanoparticle/TiO2 Aerogels for Room-Temperature Hydrolytic Decomposition of the Chemical Warfare Simulant Dimethyl Methylphosphonate
ACS Applied Nano Materials ( IF 5.3 ) Pub Date : 2020-03-26 , DOI: 10.1021/acsanm.0c00228 Monica McEntee 1 , Wesley O. Gordon 1 , Alex Balboa 1 , Daniel J. Delia 2 , Catherine L. Pitman 3 , Ashley M. Pennington 3 , Debra R. Rolison 2 , Jeremy J. Pietron 2 , Paul A. DeSario 2
ACS Applied Nano Materials ( IF 5.3 ) Pub Date : 2020-03-26 , DOI: 10.1021/acsanm.0c00228 Monica McEntee 1 , Wesley O. Gordon 1 , Alex Balboa 1 , Daniel J. Delia 2 , Catherine L. Pitman 3 , Ashley M. Pennington 3 , Debra R. Rolison 2 , Jeremy J. Pietron 2 , Paul A. DeSario 2
Affiliation
|
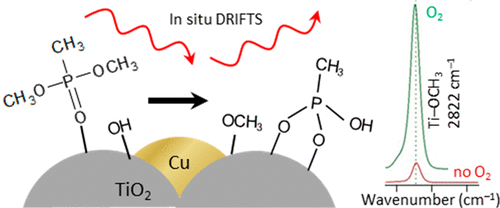
Mesoporous copper–titanium dioxide (Cu/TiO2) composite aerogels with <5-nm-diameter copper (Cu) nanoparticles hydrolyze the chemical warfare (CW) simulant dimethyl methylphosphonate (DMMP) under aerobic and anaerobic conditions. After Cu/TiO2 is exposed to DMMP in an in situ diffuse-reflectance infrared Fourier transform spectroscopy (DRIFTS) reaction chamber, hydrolysis products (i.e., methoxy groups) are bound to the surface, while no intact DMMP is observed. In contrast, DMMP degradation is not observed under our DRIFTS reactor conditions at native TiO2 aerogels, CuO, or Cu2O nanoparticles. We attribute the hydrolytic activity of Cu/TiO2 aerogels to a high surface concentration of OH species that form at Cu||TiO2 junctions. Neither hydrolysis of DMMP nor excess surface OH is observed on Au/TiO2 aerogels. The poor ability of the Au||TiO2 interface to activate water relative to that of the Cu||TiO2 interface suggests that a readily reducible supporting oxide is insufficient to promote an excess population of surface OH species—the supported nanoparticle must be sufficiently redox-active as well. Under aerobic conditions, DMMP hydrolysis is accelerated on Cu/TiO2, suggesting that O2 promotes the formation and turnover of surface OH sites. The general materials design demonstrated here of a Cu nanoparticle/reducing oxide aerogel is a promising route to CW decontamination as well other surface-mediated chemistries requiring the activation of water.
中文翻译:

介孔铜纳米颗粒/ TiO 2气凝胶用于化学战模拟物甲基膦酸二甲酯的室温水解
直径小于5纳米的铜(Cu)纳米粒子的介孔铜-二氧化钛(Cu / TiO 2)复合气凝胶在有氧和无氧条件下水解化学战(CW)模拟甲基膦酸二甲酯(DMMP)。在原位漫反射红外傅里叶变换光谱(DRIFTS)反应室内将Cu / TiO 2暴露于DMMP后,水解产物(即甲氧基)结合到表面,而未观察到完整的DMMP。相反,在我们的DRIFTS反应器条件下,在天然TiO 2气凝胶,CuO或Cu 2 O纳米颗粒上未观察到DMMP降解。我们归因于Cu / TiO 2的水解活性形成高浓度的在Cu || TiO 2结处形成的OH种类的气凝胶。在Au / TiO 2气凝胶上都没有观察到DMMP的水解和过量的表面OH 。相对于Cu || TiO 2界面,Au || TiO 2界面活化水的能力较弱,这表明易于还原的支撑氧化物不足以促进过多的表面OH物种-支撑的纳米粒子必须足够氧化还原活性。在有氧条件下,DMMP在Cu / TiO 2上的水解加速,表明O 2促进表面OH位的形成和更新。这里展示的铜纳米粒子/还原氧化物气凝胶的一般材料设计是进行CW去污以及其他需要水活化的表面介导化学方法的有前途的途径。
更新日期:2020-03-26
中文翻译:

介孔铜纳米颗粒/ TiO 2气凝胶用于化学战模拟物甲基膦酸二甲酯的室温水解
直径小于5纳米的铜(Cu)纳米粒子的介孔铜-二氧化钛(Cu / TiO 2)复合气凝胶在有氧和无氧条件下水解化学战(CW)模拟甲基膦酸二甲酯(DMMP)。在原位漫反射红外傅里叶变换光谱(DRIFTS)反应室内将Cu / TiO 2暴露于DMMP后,水解产物(即甲氧基)结合到表面,而未观察到完整的DMMP。相反,在我们的DRIFTS反应器条件下,在天然TiO 2气凝胶,CuO或Cu 2 O纳米颗粒上未观察到DMMP降解。我们归因于Cu / TiO 2的水解活性形成高浓度的在Cu || TiO 2结处形成的OH种类的气凝胶。在Au / TiO 2气凝胶上都没有观察到DMMP的水解和过量的表面OH 。相对于Cu || TiO 2界面,Au || TiO 2界面活化水的能力较弱,这表明易于还原的支撑氧化物不足以促进过多的表面OH物种-支撑的纳米粒子必须足够氧化还原活性。在有氧条件下,DMMP在Cu / TiO 2上的水解加速,表明O 2促进表面OH位的形成和更新。这里展示的铜纳米粒子/还原氧化物气凝胶的一般材料设计是进行CW去污以及其他需要水活化的表面介导化学方法的有前途的途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号