当前位置:
X-MOL 学术
›
Adv. Mater. Interfaces
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Spontaneous Adsorption of Graphene Oxide to Oil–Water and Air–Water Interfaces by Adsorption of Hydrotropes
Advanced Materials Interfaces ( IF 4.3 ) Pub Date : 2020-03-23 , DOI: 10.1002/admi.201901810 Geosmin A. Turpin 1 , Stephen A. Holt 2 , Joel M. P. Scofield 3 , Boon M. Teo 1 , Rico F. Tabor 1
Advanced Materials Interfaces ( IF 4.3 ) Pub Date : 2020-03-23 , DOI: 10.1002/admi.201901810 Geosmin A. Turpin 1 , Stephen A. Holt 2 , Joel M. P. Scofield 3 , Boon M. Teo 1 , Rico F. Tabor 1
Affiliation
|
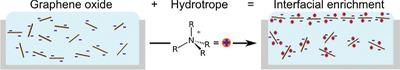
The interfacial adsorption of graphene oxide (GO) is crucial in phenomena such as emulsification and froth flotation, where presence of 2D nanomaterials facilitates Pickering stabilization. This process usually requires the input of high amounts of shear energy, or is aided by surfactants in order to make it possible at room temperature. In this work, a surfactant‐free method for interfacial enrichment through the use of a family of tetraalkylammonium hydrotropes, the most effective being tetraethylammonium chloride (TEAC), is demonstrated. As both GO and hydrotropes do not spontaneously enrich to interfaces on their own, this synergistic, spontaneous effect highlights that hydrotropes adsorb to GO sheets, decreasing their negative charge while rendering them more amphiphilic and therefore making it thermodynamically favorable for them to adsorb to the interface. Evidence for this adsorption includes increases in surface pressure, as well as emulsion and froth stability when both GO and hydrotropes are present in a system. Hydrotropes perform as well as or better than surfactants. Adsorption is irreversible, with XRR and AFM studies demonstrating that roughness increases with compression of the air‐water interface, showing that GO sheets are crumpling at the interface rather than desorbing, providing new routes to patterned and structured GO layers.
中文翻译:

水溶助长剂自发氧化石墨烯在油-水和空气-水界面的吸附
氧化石墨烯(GO)的界面吸附在诸如乳化和泡沫浮选等现象中至关重要,其中2D纳米材料的存在有助于Pickering稳定化。该方法通常需要输入大量的剪切能,或在表面活性剂的辅助下使其在室温下成为可能。在这项工作中,证明了通过使用四烷基铵水溶助长剂家族(一种最有效的是氯化四乙基铵)来进行界面富集的无表面活性剂的方法。由于GO和水溶助长剂都不会自行自发地富集到界面上,因此这种协同的自发效应表明水溶助长剂会吸附到GO片材上,减少它们的负电荷,同时使其更两亲,因此使其在热力学上有利于它们吸附到界面上。当系统中同时存在GO和水溶助长剂时,这种吸附的证据包括表面压力的增加以及乳液和泡沫的稳定性。水溶助长剂的性能与表面活性剂相同或更好。吸附是不可逆的,XRR和AFM研究表明,粗糙度随着空气-水界面的压缩而增加,表明GO片材在界面处起皱而不是解吸,这为图案化和结构化GO层提供了新的途径。水溶助长剂的性能与表面活性剂相同或更好。吸附是不可逆的,XRR和AFM研究表明,粗糙度随着空气-水界面的压缩而增加,表明GO片材在界面处起皱而不是解吸,这为图案化和结构化GO层提供了新的途径。水溶助长剂的性能与表面活性剂相同或更好。吸附是不可逆的,XRR和AFM研究表明,粗糙度随空气-水界面的压缩而增加,表明GO片材在界面处起皱而不是解吸,这为图案化和结构化GO层提供了新途径。
更新日期:2020-03-23
中文翻译:

水溶助长剂自发氧化石墨烯在油-水和空气-水界面的吸附
氧化石墨烯(GO)的界面吸附在诸如乳化和泡沫浮选等现象中至关重要,其中2D纳米材料的存在有助于Pickering稳定化。该方法通常需要输入大量的剪切能,或在表面活性剂的辅助下使其在室温下成为可能。在这项工作中,证明了通过使用四烷基铵水溶助长剂家族(一种最有效的是氯化四乙基铵)来进行界面富集的无表面活性剂的方法。由于GO和水溶助长剂都不会自行自发地富集到界面上,因此这种协同的自发效应表明水溶助长剂会吸附到GO片材上,减少它们的负电荷,同时使其更两亲,因此使其在热力学上有利于它们吸附到界面上。当系统中同时存在GO和水溶助长剂时,这种吸附的证据包括表面压力的增加以及乳液和泡沫的稳定性。水溶助长剂的性能与表面活性剂相同或更好。吸附是不可逆的,XRR和AFM研究表明,粗糙度随着空气-水界面的压缩而增加,表明GO片材在界面处起皱而不是解吸,这为图案化和结构化GO层提供了新的途径。水溶助长剂的性能与表面活性剂相同或更好。吸附是不可逆的,XRR和AFM研究表明,粗糙度随着空气-水界面的压缩而增加,表明GO片材在界面处起皱而不是解吸,这为图案化和结构化GO层提供了新的途径。水溶助长剂的性能与表面活性剂相同或更好。吸附是不可逆的,XRR和AFM研究表明,粗糙度随空气-水界面的压缩而增加,表明GO片材在界面处起皱而不是解吸,这为图案化和结构化GO层提供了新途径。











































 京公网安备 11010802027423号
京公网安备 11010802027423号