当前位置:
X-MOL 学术
›
Aging Cell
›
论文详情
Our official English website, www.x-mol.net, welcomes your feedback! (Note: you will need to create a separate account there.)
Deterioration of hematopoietic autophagy is linked to osteoporosis.
Aging Cell ( IF 7.8 ) Pub Date : 2020-03-25 , DOI: 10.1111/acel.13114 Ye Yuan 1, 2 , Yixuan Fang 3, 4, 5 , Lingjiang Zhu 3 , Yue Gu 3 , Lei Li 3 , Jiawei Qian 3 , Ruijin Zhao 3 , Peng Zhang 1, 2 , Jian Li 1, 2 , Hui Zhang 1, 2 , Na Yuan 3, 4, 5 , Suping Zhang 3, 4, 5 , Quanhong Ma 5 , Jianrong Wang 3, 4, 5 , Youjia Xu 1, 2
Aging Cell ( IF 7.8 ) Pub Date : 2020-03-25 , DOI: 10.1111/acel.13114 Ye Yuan 1, 2 , Yixuan Fang 3, 4, 5 , Lingjiang Zhu 3 , Yue Gu 3 , Lei Li 3 , Jiawei Qian 3 , Ruijin Zhao 3 , Peng Zhang 1, 2 , Jian Li 1, 2 , Hui Zhang 1, 2 , Na Yuan 3, 4, 5 , Suping Zhang 3, 4, 5 , Quanhong Ma 5 , Jianrong Wang 3, 4, 5 , Youjia Xu 1, 2
Affiliation
|
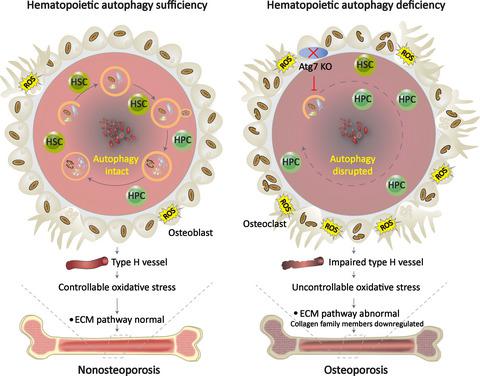
Hematopoietic disorders are known to increase the risk of complications such as osteoporosis. However, a direct link between hematopoietic cellular disorders and osteoporosis has been elusive. Here, we demonstrate that the deterioration of hematopoietic autophagy is coupled with osteoporosis in humans. With a conditional mouse model in which autophagy in the hematopoietic system is disrupted by deletion of the Atg7 gene, we show that incapacitating hematopoietic autophagy causes bone loss and perturbs osteocyte homeostasis. Induction of osteoporosis, either by ovariectomy, which blocks estrogen secretion, or by injection of ferric ammonium citrate to induce iron overload, causes dysfunction in the hematopoietic stem and progenitor cells (HSPCs) similar to that found in autophagy‐defective mice. Transcriptomic analysis of HSPCs suggests promotion of iron activity and inhibition of osteocyte differentiation and calcium metabolism by hematopoietic autophagy defect, while proteomic profiling of bone tissue proteins indicates disturbance of the extracellular matrix pathway that includes collagen family members. Finally, screening for expression of selected genes and an immunohistological assay identifies severe impairments in H vessels in the bone tissue, which results in disconnection of osteocytes from hematopoietic cells in the autophagy‐defective mice. We therefore propose that hematopoietic autophagy is required for the integrity of H vessels that bridge blood and bone cells and that its deterioration leads to osteoporosis.
中文翻译:

造血自噬的恶化与骨质疏松症有关。
已知造血功能障碍会增加并发症(如骨质疏松症)的风险。然而,造血细胞疾病与骨质疏松症之间的直接联系一直难以捉摸。在这里,我们证明造血自噬的恶化与人类的骨质疏松症相关。在条件小鼠模型中,通过删除Atg7基因破坏了造血系统中的自噬,我们证明了丧失能力的造血自噬会导致骨丢失并扰动骨细胞稳态。卵巢切除术会阻断雌激素分泌,或者注射柠檬酸铁铵以诱导铁超负荷,导致骨质疏松症引起造血干细胞和祖细胞(HSPC)功能异常,类似于自噬缺陷小鼠中的情况。HSPC的转录组学分析表明,造血自噬缺陷促进了铁的活性,抑制了骨细胞的分化和钙代谢,而骨组织蛋白的蛋白质组学分析表明包括胶原蛋白家族成员在内的细胞外基质途径受到干扰。最后,筛选选定基因的表达并进行免疫组织学分析,鉴定骨组织中H血管的严重损伤,这导致自噬缺陷小鼠的骨细胞与造血细胞脱离。因此,我们提出造血自噬是桥接血液和骨细胞的H血管的完整性所必需的,并且其恶化会导致骨质疏松。骨组织蛋白的蛋白质组学分析表明包括胶原蛋白家族成员的细胞外基质途径受到干扰。最后,筛选选定基因的表达并进行免疫组织学分析,鉴定骨组织中H血管的严重损伤,这导致自噬缺陷小鼠的骨细胞与造血细胞脱离。因此,我们提出造血自噬是桥接血液和骨细胞的H血管的完整性所必需的,并且其恶化会导致骨质疏松。骨组织蛋白的蛋白质组学分析表明包括胶原蛋白家族成员的细胞外基质途径受到干扰。最后,通过筛选选定基因的表达并进行免疫组织化学分析,可以确定骨组织中H血管的严重损伤,从而导致自噬缺陷小鼠的骨细胞与造血细胞分离。因此,我们提出造血自噬是桥接血液和骨细胞的H血管的完整性所必需的,并且其恶化会导致骨质疏松。筛选选定基因的表达并进行免疫组织化学分析,鉴定骨组织中H血管的严重损伤,从而导致自噬缺陷小鼠的骨细胞与造血细胞分离。因此,我们提出造血自噬是桥接血液和骨细胞的H血管的完整性所必需的,并且其恶化会导致骨质疏松。筛选选定基因的表达并进行免疫组织化学分析,鉴定骨组织中H血管的严重损伤,从而导致自噬缺陷小鼠的骨细胞与造血细胞分离。因此,我们提出造血自噬是桥接血液和骨细胞的H血管的完整性所必需的,并且其恶化会导致骨质疏松。
更新日期:2020-03-25
中文翻译:

造血自噬的恶化与骨质疏松症有关。
已知造血功能障碍会增加并发症(如骨质疏松症)的风险。然而,造血细胞疾病与骨质疏松症之间的直接联系一直难以捉摸。在这里,我们证明造血自噬的恶化与人类的骨质疏松症相关。在条件小鼠模型中,通过删除Atg7基因破坏了造血系统中的自噬,我们证明了丧失能力的造血自噬会导致骨丢失并扰动骨细胞稳态。卵巢切除术会阻断雌激素分泌,或者注射柠檬酸铁铵以诱导铁超负荷,导致骨质疏松症引起造血干细胞和祖细胞(HSPC)功能异常,类似于自噬缺陷小鼠中的情况。HSPC的转录组学分析表明,造血自噬缺陷促进了铁的活性,抑制了骨细胞的分化和钙代谢,而骨组织蛋白的蛋白质组学分析表明包括胶原蛋白家族成员在内的细胞外基质途径受到干扰。最后,筛选选定基因的表达并进行免疫组织学分析,鉴定骨组织中H血管的严重损伤,这导致自噬缺陷小鼠的骨细胞与造血细胞脱离。因此,我们提出造血自噬是桥接血液和骨细胞的H血管的完整性所必需的,并且其恶化会导致骨质疏松。骨组织蛋白的蛋白质组学分析表明包括胶原蛋白家族成员的细胞外基质途径受到干扰。最后,筛选选定基因的表达并进行免疫组织学分析,鉴定骨组织中H血管的严重损伤,这导致自噬缺陷小鼠的骨细胞与造血细胞脱离。因此,我们提出造血自噬是桥接血液和骨细胞的H血管的完整性所必需的,并且其恶化会导致骨质疏松。骨组织蛋白的蛋白质组学分析表明包括胶原蛋白家族成员的细胞外基质途径受到干扰。最后,通过筛选选定基因的表达并进行免疫组织化学分析,可以确定骨组织中H血管的严重损伤,从而导致自噬缺陷小鼠的骨细胞与造血细胞分离。因此,我们提出造血自噬是桥接血液和骨细胞的H血管的完整性所必需的,并且其恶化会导致骨质疏松。筛选选定基因的表达并进行免疫组织化学分析,鉴定骨组织中H血管的严重损伤,从而导致自噬缺陷小鼠的骨细胞与造血细胞分离。因此,我们提出造血自噬是桥接血液和骨细胞的H血管的完整性所必需的,并且其恶化会导致骨质疏松。筛选选定基因的表达并进行免疫组织化学分析,鉴定骨组织中H血管的严重损伤,从而导致自噬缺陷小鼠的骨细胞与造血细胞分离。因此,我们提出造血自噬是桥接血液和骨细胞的H血管的完整性所必需的,并且其恶化会导致骨质疏松。



























 京公网安备 11010802027423号
京公网安备 11010802027423号