当前位置:
X-MOL 学术
›
ChemPhysChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
An Extensive Set of Accurate Fluoride Ion Affinities for p-Block Element Lewis Acids and Basic Design Principles for Strong Fluoride Ion Acceptors.
ChemPhysChem ( IF 2.3 ) Pub Date : 2020-04-20 , DOI: 10.1002/cphc.202000244 Philipp Erdmann 1 , Jonas Leitner 1 , Julia Schwarz 1 , Lutz Greb 1
ChemPhysChem ( IF 2.3 ) Pub Date : 2020-04-20 , DOI: 10.1002/cphc.202000244 Philipp Erdmann 1 , Jonas Leitner 1 , Julia Schwarz 1 , Lutz Greb 1
Affiliation
|
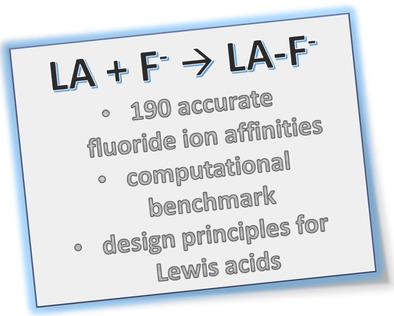
The computed fluoride ion affinity (FIA) is a valuable descriptor to assess the Lewis acidity of a compound. Despite its widespread use, the varying accuracy of applied computational models hampers the broad comparability of literature data. Herein, we evaluate the performance of selected methods (like DLPNO‐CCSD(T)) in FIA computations against CCSD(T)/CBS data and guide for the choice of suitable density functionals that allow the treatment of larger Lewis acids. Based on the benchmarked methods, we computed an extensive set of gas‐phase and solvation corrected FIA, that is covering group 13–16 elements featuring moderate to strong electron‐withdrawing substituents (190 entries). It permits an unbiased comparison of FIA over a significant fraction of the periodic table, serves as a source of reference for future synthetic or theoretical studies, and allows to derive some simple design principles for strong fluoride ion acceptors. Finally, the manuscript includes a tutorial section for the computation of FIA with and without the consideration of solvation.
中文翻译:

对p嵌段元素路易斯酸的大量精确的氟离子亲和力以及强氟离子受体的基本设计原理。
计算得出的氟离子亲和力(FIA)是评估化合物路易斯酸度的有价值的描述符。尽管已广泛使用,但应用计算模型的准确性各不相同,这妨碍了文献数据的广泛可比性。在本文中,我们针对FCC计算中的CCSD(T)/ CBS数据评估了所选方法(如DLPNO-CCSD(T))的性能,并为选择合适的密度官能团(可处理较大的路易斯酸)提供了指南。基于基准测试方法,我们计算了一组广泛的气相和溶剂化校正的FIA,涵盖了具有中至强吸电子取代基(190个条目)的13-16组元素。它可以在大部分周期表中对FIA进行无偏比较,并为将来的综合或理论研究提供参考,并允许得出一些简单的设计原理,用于强氟离子受体。最后,该手稿包括一个在不考虑溶剂化的情况下计算FIA的教程部分。
更新日期:2020-04-20
中文翻译:

对p嵌段元素路易斯酸的大量精确的氟离子亲和力以及强氟离子受体的基本设计原理。
计算得出的氟离子亲和力(FIA)是评估化合物路易斯酸度的有价值的描述符。尽管已广泛使用,但应用计算模型的准确性各不相同,这妨碍了文献数据的广泛可比性。在本文中,我们针对FCC计算中的CCSD(T)/ CBS数据评估了所选方法(如DLPNO-CCSD(T))的性能,并为选择合适的密度官能团(可处理较大的路易斯酸)提供了指南。基于基准测试方法,我们计算了一组广泛的气相和溶剂化校正的FIA,涵盖了具有中至强吸电子取代基(190个条目)的13-16组元素。它可以在大部分周期表中对FIA进行无偏比较,并为将来的综合或理论研究提供参考,并允许得出一些简单的设计原理,用于强氟离子受体。最后,该手稿包括一个在不考虑溶剂化的情况下计算FIA的教程部分。











































 京公网安备 11010802027423号
京公网安备 11010802027423号