当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
One‐Pot Synthesis of Densely Substituted 1,2,3,4‐Tetrahydro‐1,6‐naphthyridine Mediated by Isocyanide‐Assisted Reduction of C−C Double Bond
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-03-25 , DOI: 10.1002/slct.202000441 Paramita Das 1, 2 , Suman Ray 1, 3 , Rupak Saha 4 , Chhanda Mukhopadhyay 1
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-03-25 , DOI: 10.1002/slct.202000441 Paramita Das 1, 2 , Suman Ray 1, 3 , Rupak Saha 4 , Chhanda Mukhopadhyay 1
Affiliation
|
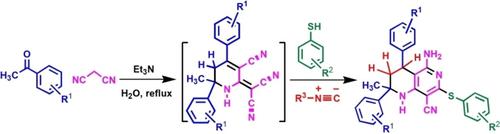
Molecular architectures possessing the combination of heteroaromatic and saturated N‐heterocycles are of great importance because of their higher solubility in the gastrointestinal tract due to weak crystal packing in the three‐dimensional structure. Other biological activity like selectivity is also increased in a positive way. However, compared to fully aromatic fused heterocycles, synthesis of partially saturated fused heterocycles is much more difficult since the later needs greater control over the reaction conditions. In this context, 1,2,3,4‐tetrahydronaphthyridines (THNADs) are essential part of pharmaceutically important natural products and drug molecules. However, the synthesis of THNAD is seldom reported in literature. To the best of our knowledge, this is the first report of metal‐free one pot synthesis of 1,2,3,4‐tetrahydro‐1,6‐naphthyridines without starting from any nitrogen heterocycles in water. Moreover, this study discloses the involvement of isocyanide in a chemical reaction whose net effect is only to reduce a C=C bond which is unusual in isocyanide literature.
中文翻译:

异氰化物辅助还原C-C双键介导的重取代的1,2,3,4-四氢-1,6-萘啶的一锅合成
具有杂芳族和饱和N杂环的组合的分子结构非常重要,因为由于三维结构中的晶体堆积较弱,它们在胃肠道中的溶解度更高。其他生物活性(如选择性)也以积极的方式增加。但是,与完全芳族稠合的杂环相比,部分饱和的稠合杂环的合成要困难得多,因为后者需要更好地控制反应条件。在这种情况下,1,2,3,4-四氢萘啶(THNAD)是重要的天然药物和药物分子的重要组成部分。然而,文献中很少报道THNAD的合成。据我们所知,这是关于1,2,3,4-四氢-1,6-萘啶,无需从水中的任何氮杂环开始。而且,该研究公开了异氰酸酯参与化学反应,其净作用仅是还原C = C键,这在异氰酸酯文献中是不常见的。
更新日期:2020-03-26
中文翻译:

异氰化物辅助还原C-C双键介导的重取代的1,2,3,4-四氢-1,6-萘啶的一锅合成
具有杂芳族和饱和N杂环的组合的分子结构非常重要,因为由于三维结构中的晶体堆积较弱,它们在胃肠道中的溶解度更高。其他生物活性(如选择性)也以积极的方式增加。但是,与完全芳族稠合的杂环相比,部分饱和的稠合杂环的合成要困难得多,因为后者需要更好地控制反应条件。在这种情况下,1,2,3,4-四氢萘啶(THNAD)是重要的天然药物和药物分子的重要组成部分。然而,文献中很少报道THNAD的合成。据我们所知,这是关于1,2,3,4-四氢-1,6-萘啶,无需从水中的任何氮杂环开始。而且,该研究公开了异氰酸酯参与化学反应,其净作用仅是还原C = C键,这在异氰酸酯文献中是不常见的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号