当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
6,7‐Dihydro‐5H‐1,2,4‐triazolo[3,4‐b][1,3,4]thiadiazine Ring Cleavage and Tautomerism of the Products: Experimental and Theoretical Study
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-03-25 , DOI: 10.1002/slct.202000732 Alexandra A. Kolodina 1 , Arshak A. Tsaturyan 1 , Maria S. Galkina 1 , Inna G. Borodkina 1 , Elena V. Vetrova 1 , Oleg P. Demidov 2 , Alexandra G. Berezhnaya 3 , Anatoly V. Metelitsa 1
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-03-25 , DOI: 10.1002/slct.202000732 Alexandra A. Kolodina 1 , Arshak A. Tsaturyan 1 , Maria S. Galkina 1 , Inna G. Borodkina 1 , Elena V. Vetrova 1 , Oleg P. Demidov 2 , Alexandra G. Berezhnaya 3 , Anatoly V. Metelitsa 1
Affiliation
|
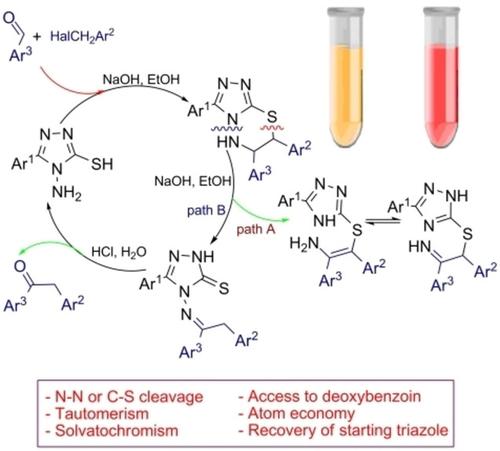
Electronic effects of aryl substituents at the C(7)‐atom play a crucial role in the cleavage of a six‐membered ring of 6,7‐dihydro‐5H‐[1,2,4]triazolo[3,4‐b][1,3,4]thiadiazines. Our experiments show that electron‐withdrawing 2‐nitrophenyl and 4‐nitrophenyl groups promote the N−N‐bond cleavage, whereas a phenyl group and a bulky 4,5‐dimethoxy‐2‐nitrophenyl substituent are favored for the C−S‐bond cleavage, affording novel triazole derivatives. UV‐vis, electrochemical, and dynamic NMR studies as well as DFT calculations of product's tautomeric equilibria were performed.
中文翻译:

6,7-二氢-5H-1,2,4-三唑并[3,4-b] [1,3,4]噻二嗪环的裂解和互变异构:实验和理论研究
在C(7) -原子的芳基取代基电子效应在6,7-二氢-5-一个六元环的裂解中发挥关键作用ħ [1,2,4]三唑并[3,4 - b ] [1,3,4]噻二嗪。我们的实验表明,吸电子的2-硝基苯基和4-硝基苯基促进了N-N-键的裂解,而苯基和庞大的4,5-二甲氧基-2-硝基苯基取代基更适合于CS键裂解,得到新的三唑衍生物。进行了紫外可见,电化学和动态NMR研究,以及产品互变异构平衡的DFT计算。
更新日期:2020-03-26
中文翻译:

6,7-二氢-5H-1,2,4-三唑并[3,4-b] [1,3,4]噻二嗪环的裂解和互变异构:实验和理论研究
在C(7) -原子的芳基取代基电子效应在6,7-二氢-5-一个六元环的裂解中发挥关键作用ħ [1,2,4]三唑并[3,4 - b ] [1,3,4]噻二嗪。我们的实验表明,吸电子的2-硝基苯基和4-硝基苯基促进了N-N-键的裂解,而苯基和庞大的4,5-二甲氧基-2-硝基苯基取代基更适合于CS键裂解,得到新的三唑衍生物。进行了紫外可见,电化学和动态NMR研究,以及产品互变异构平衡的DFT计算。











































 京公网安备 11010802027423号
京公网安备 11010802027423号