当前位置:
X-MOL 学术
›
ChemistrySelect
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Interaction of Water at the Hydrophobic Interface of Alkyl Group of Alcohol with p‐Nitro‐Aniline Charge Transfer State
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-03-25 , DOI: 10.1002/slct.201904476 Smruti Ranjana Parida 1 , Himansu Mohapatra 1 , Snigdhashree Priyadarshini 1
ChemistrySelect ( IF 1.9 ) Pub Date : 2020-03-25 , DOI: 10.1002/slct.201904476 Smruti Ranjana Parida 1 , Himansu Mohapatra 1 , Snigdhashree Priyadarshini 1
Affiliation
|
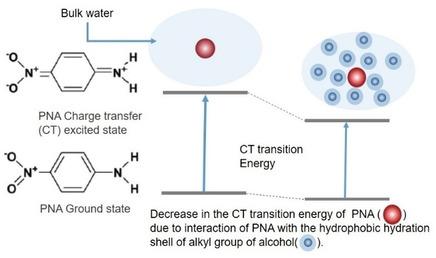
The evolution of photo‐induced intra molecular charge transfer (ICT) transition energy of p‐nitro‐aniline (PNA), with respect to the mole fraction of alcohol in alcohol water mixture has been used to study, the interaction of hydrophobic hydration ‐shell of linear alcohols with PNA‐ICT state. The linear alcohol ranges from methanol to propanol. The plot of absorption energy of PNA charge transfer transition versus mole fraction of alcohol shows the following features. At lower mole fraction of alcohol, the absorption energy of PNA versus mole‐fraction of alcohol plot at first, decreases below the absorption energy of PNA recorded in pure water and alcohol, goes through a minimum and then increases. Similar behaviour is obtained even after subtracting the contribution from dielectric non‐ideality. The behaviour at lower mole fraction thus indicates the modification of specific interaction of PNA with water due to the presence of alcohol. Further, the absorption energy minimum shifts towards lower mole fraction of alcohol as the chain length of alcohol increases. These results, thus reveals the interaction of PNA with the modified water structure at the hydrophobic interface of alkyl group and supports the current understanding of hydrophobic hydration. To our knowledge, this study for the first time reveals that, hydration shell around hydrophobic group has higher ability to stabilize the PNA‐ICT state by hydrogen bonding interaction compared to bulk water.
中文翻译:

水在醇的烷基疏水界面与对硝基苯胺电荷转移态的相互作用
p的光致分子内电荷转移(ICT)跃迁能的演化关于硝基苯胺(PNA),相对于醇与水混合物中的醇的摩尔分数,已经用于研究线性醇的疏水水合壳与PNA-ICT状态的相互作用。直链醇的范围从甲醇到丙醇。PNA电荷转移跃迁的吸收能与醇的摩尔分数的关系图显示出以下特征。在较低的酒精摩尔分数下,PNA的吸收能量相对于酒精的摩尔分数首先下降,低于纯净水和酒精中记录的PNA的吸收能量,经过最小然后增加。即使从介电非理想中减去贡献,也可以获得类似的行为。因此,在较低摩尔分数下的行为表明由于醇的存在,PNA与水的特异性相互作用的改变。此外,随着醇的链长的增加,吸收能的最小值向醇的较低摩尔分数移动。这些结果因此揭示了PNA与烷基疏水界面处的改性水结构之间的相互作用,并支持了目前对疏水水合的理解。据我们所知,这项研究首次表明,与大量水相比,疏水基团周围的水合壳通过氢键相互作用具有更高的稳定PNA-ICT状态的能力。
更新日期:2020-03-26
中文翻译:

水在醇的烷基疏水界面与对硝基苯胺电荷转移态的相互作用
p的光致分子内电荷转移(ICT)跃迁能的演化关于硝基苯胺(PNA),相对于醇与水混合物中的醇的摩尔分数,已经用于研究线性醇的疏水水合壳与PNA-ICT状态的相互作用。直链醇的范围从甲醇到丙醇。PNA电荷转移跃迁的吸收能与醇的摩尔分数的关系图显示出以下特征。在较低的酒精摩尔分数下,PNA的吸收能量相对于酒精的摩尔分数首先下降,低于纯净水和酒精中记录的PNA的吸收能量,经过最小然后增加。即使从介电非理想中减去贡献,也可以获得类似的行为。因此,在较低摩尔分数下的行为表明由于醇的存在,PNA与水的特异性相互作用的改变。此外,随着醇的链长的增加,吸收能的最小值向醇的较低摩尔分数移动。这些结果因此揭示了PNA与烷基疏水界面处的改性水结构之间的相互作用,并支持了目前对疏水水合的理解。据我们所知,这项研究首次表明,与大量水相比,疏水基团周围的水合壳通过氢键相互作用具有更高的稳定PNA-ICT状态的能力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号