当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Ruthenium(II)-Catalyzed Double Annulation of Quinones: Step-Economical Access to Valuable Bioactive Compounds.
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-03-25 , DOI: 10.1002/chem.202001434 Eufrânio N da Silva Júnior 1, 2 , Renato L de Carvalho 1, 2 , Renata G Almeida 2 , Luisa G Rosa 2 , Felipe Fantuzzi 3 , Torben Rogge 1 , Pedro M S Costa 4 , Claudia Pessoa 4 , Claus Jacob 5 , Lutz Ackermann 1, 6
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-03-25 , DOI: 10.1002/chem.202001434 Eufrânio N da Silva Júnior 1, 2 , Renato L de Carvalho 1, 2 , Renata G Almeida 2 , Luisa G Rosa 2 , Felipe Fantuzzi 3 , Torben Rogge 1 , Pedro M S Costa 4 , Claudia Pessoa 4 , Claus Jacob 5 , Lutz Ackermann 1, 6
Affiliation
|
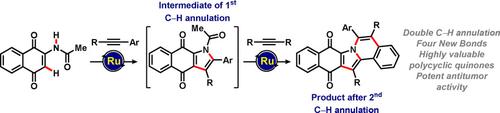
Double ruthenium(II)‐catalyzed alkyne annulations of quinones were accomplished. Thus, a strategy is reported that provides step‐economical access to valuable quinones with a wide range of applications. C−H/N−H activations for alkyne annulations of naphthoquinones provided challenging polycyclic quinoidal compounds by forming four new bonds in one step. The singular power of the thus‐obtained compounds was reflected by their antileukemic activity.
中文翻译:

钌(II)催化的醌双环:对重要生物活性化合物的逐步经济获取。
完成了双钌(II)催化的炔烃炔环。因此,据报道,一种策略可以经济实用地提供有价值的醌,并具有广泛的应用范围。萘醌炔烃环化的C-H / N-H活化通过一步形成四个新键,提供了具有挑战性的多环喹啉化合物。如此获得的化合物的非凡能力通过其抗白血病活性反映出来。
更新日期:2020-03-25
中文翻译:

钌(II)催化的醌双环:对重要生物活性化合物的逐步经济获取。
完成了双钌(II)催化的炔烃炔环。因此,据报道,一种策略可以经济实用地提供有价值的醌,并具有广泛的应用范围。萘醌炔烃环化的C-H / N-H活化通过一步形成四个新键,提供了具有挑战性的多环喹啉化合物。如此获得的化合物的非凡能力通过其抗白血病活性反映出来。










































 京公网安备 11010802027423号
京公网安备 11010802027423号