当前位置:
X-MOL 学术
›
Chem. Eur. J.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
CpTiCl2 -Catalyzed Cross-Coupling between Internal Alkynes and Ketones: A Novel Concept in the Synthesis of Halogenated, Conjugated Dienes.
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-03-25 , DOI: 10.1002/chem.202001023 Esther Roldan-Molina 1 , Maria M Nievas 1 , Jorge A R Navarro 2 , J Enrique Oltra 1
Chemistry - A European Journal ( IF 3.9 ) Pub Date : 2020-03-25 , DOI: 10.1002/chem.202001023 Esther Roldan-Molina 1 , Maria M Nievas 1 , Jorge A R Navarro 2 , J Enrique Oltra 1
Affiliation
|
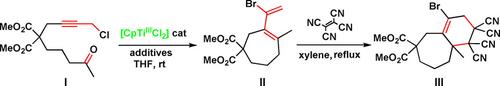
A novel concept for the synthesis of halogenated, conjugated dienes is disclosed: the CpTiCl2‐catalyzed coupling of keto‐alkynes, in the presence of Me3SiBr/Et3N⋅ HBr. This reaction provided five‐, six‐, and seven‐membered carbocycles, nitrogenated heterocycles, as well as six‐membered oxygenated heterocycles leading to a brominated conjugate diene. These products showed high reactivity in the Diels–Alder, Suzuki, and Sonogashira reactions, giving complex chemical structures in only three steps from the corresponding acyclic keto‐alkyne. Hopefully, this strategy will pave the way towards the synthesis of bioactive natural products and new materials.
中文翻译:

CpTiCl2催化的内部炔烃和酮之间的交叉偶联:卤代共轭二烯的合成中的新概念。
用于卤化的,共轭二烯的合成了一种新的概念是公开:所述CpTiCl 2酮-炔烃的催化的偶联,在我的存在3 SIBR / ET 3 Ñ ⋅的HBr。该反应提供了五元,六元和七元碳环,含氮杂环以及六元含氧杂环,从而生成溴化共轭二烯。这些产物在Diels-Alder,Suzuki和Sonogashira反应中显示出高反应活性,与相应的无环酮炔烃仅三步即可得到复杂的化学结构。希望这一策略将为生物活性天然产物和新材料的合成铺平道路。
更新日期:2020-03-25
中文翻译:

CpTiCl2催化的内部炔烃和酮之间的交叉偶联:卤代共轭二烯的合成中的新概念。
用于卤化的,共轭二烯的合成了一种新的概念是公开:所述CpTiCl 2酮-炔烃的催化的偶联,在我的存在3 SIBR / ET 3 Ñ ⋅的HBr。该反应提供了五元,六元和七元碳环,含氮杂环以及六元含氧杂环,从而生成溴化共轭二烯。这些产物在Diels-Alder,Suzuki和Sonogashira反应中显示出高反应活性,与相应的无环酮炔烃仅三步即可得到复杂的化学结构。希望这一策略将为生物活性天然产物和新材料的合成铺平道路。











































 京公网安备 11010802027423号
京公网安备 11010802027423号