当前位置:
X-MOL 学术
›
CrystEngComm
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Role of intermolecular interactions and conformational changes in the polymorphism and vitrification process of 2,2′′-bis-substituted para-terphenyls
CrystEngComm ( IF 2.6 ) Pub Date : 2020-03-25 , DOI: 10.1039/d0ce00351d Andrzej Nowok 1, 2, 3, 4 , Piotr Kuś 2, 4, 5, 6 , Mateusz Dulski 2, 3, 4, 7 , Joachim Kusz 1, 2, 3, 4 , Maria Książek 1, 2, 3, 4 , Maciej Zubko 2, 3, 4, 7, 8 , Anna Z. Szeremeta 1, 2, 3, 4 , Sebastian Pawlus 1, 2, 3, 4
CrystEngComm ( IF 2.6 ) Pub Date : 2020-03-25 , DOI: 10.1039/d0ce00351d Andrzej Nowok 1, 2, 3, 4 , Piotr Kuś 2, 4, 5, 6 , Mateusz Dulski 2, 3, 4, 7 , Joachim Kusz 1, 2, 3, 4 , Maria Książek 1, 2, 3, 4 , Maciej Zubko 2, 3, 4, 7, 8 , Anna Z. Szeremeta 1, 2, 3, 4 , Sebastian Pawlus 1, 2, 3, 4
Affiliation
|
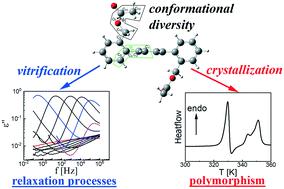
In the past decades, para-terphenyls have been attracting tremendous attention due to their polymorphism and conformational diversity. In this work we report the synthesis, crystal structure, polymorphism and dielectric properties of two 2,2′′-bis-substituted para-terphenyls: 2,2′′-bis(hydroxymethyl)-para-terphenyl and 2,2′′-bis(acetyloxymethyl)-para-terphenyl. On the basis of calorimetric and X-ray studies, we showed that the latter compound occurs in at least four polymorphic forms with melting points equal to 364, 345, 341 and 326 K, differentiated also in terms of thermodynamic stability and crystal symmetry. The most stable polymorph I is characterized by the P21/n space group. 2,2′′-Bis(hydroxymethyl)-para-terphenyl crystallizes in the monoclinic P21/c space group. Both 2,2′′-bis-substituted para-terphenyls can undergo vitrification, which is a highly exceptional feature for this class of chemical compounds and has not been reported before. Consequently, the molecular dynamics and conformational changes in the glassy and supercooled liquid states were analyzed by means of IR and broadband dielectric spectroscopy. Two relaxation processes were observed for both compounds: structural α-relaxation, connected with reorientational motions of molecules in supercooled liquid, and intermolecular γ-relaxation, ascribed to rotational motions of substituents of the para-terphenyl skeleton. Taking into account the ongoing discussion about the conformational diversity of the para-terphenyl skeleton, we showed that although free rotation of benzene rings is suppressed, the molecules in the glassy and liquid states can adopt both twisted and helical conformations, which results in diversity of the polymorphic forms.
中文翻译:

分子间相互作用和构象变化在2,2''-双取代对三联苯的多态性和玻璃化过程中的作用
在过去的几十年中,对-三联苯由于其多态性和构象多样性而引起了极大的关注。在这项工作中,我们报告了两个2,2''-双取代的对-三联苯的合成,晶体结构,多态性和介电性能:2,2''-双(羟甲基)-对-三联苯和2,2'' -双(乙酰氧基甲基)-对-三联苯。根据量热法和X射线研究,我们显示了后者的化合物至少以四种多晶型形式存在,其熔点分别等于364、345、341和326 K,并且在热力学稳定性和晶体对称性方面也有所区别。最稳定的多晶型物I的特征是P 2 1 / n空间群。2,2'-双(羟甲基)-对-三联苯在单斜晶P 2 1 / c空间基团中结晶。两个2,2'-双取代的对-三联苯都可以进行玻璃化,这是这类化合物的一个非常特殊的功能,以前从未有过报道。因此,通过红外和宽带介电谱分析了玻璃态和过冷液态的分子动力学和构象变化。两种化合物都观察到两个弛豫过程:结构α松弛与分子在过冷液体中的重新定向运动有关,以及分子间γ松弛,归因于化合物的取代基的旋转运动。对-三苯基骨架。考虑到正在进行的对-对-苯基骨架构象多样性的讨论,我们表明,尽管苯环的自由旋转受到抑制,但处于玻璃态和液态的分子可以同时采用扭曲和螺旋构象,从而导致多态形式。
更新日期:2020-03-25
中文翻译:

分子间相互作用和构象变化在2,2''-双取代对三联苯的多态性和玻璃化过程中的作用
在过去的几十年中,对-三联苯由于其多态性和构象多样性而引起了极大的关注。在这项工作中,我们报告了两个2,2''-双取代的对-三联苯的合成,晶体结构,多态性和介电性能:2,2''-双(羟甲基)-对-三联苯和2,2'' -双(乙酰氧基甲基)-对-三联苯。根据量热法和X射线研究,我们显示了后者的化合物至少以四种多晶型形式存在,其熔点分别等于364、345、341和326 K,并且在热力学稳定性和晶体对称性方面也有所区别。最稳定的多晶型物I的特征是P 2 1 / n空间群。2,2'-双(羟甲基)-对-三联苯在单斜晶P 2 1 / c空间基团中结晶。两个2,2'-双取代的对-三联苯都可以进行玻璃化,这是这类化合物的一个非常特殊的功能,以前从未有过报道。因此,通过红外和宽带介电谱分析了玻璃态和过冷液态的分子动力学和构象变化。两种化合物都观察到两个弛豫过程:结构α松弛与分子在过冷液体中的重新定向运动有关,以及分子间γ松弛,归因于化合物的取代基的旋转运动。对-三苯基骨架。考虑到正在进行的对-对-苯基骨架构象多样性的讨论,我们表明,尽管苯环的自由旋转受到抑制,但处于玻璃态和液态的分子可以同时采用扭曲和螺旋构象,从而导致多态形式。









































 京公网安备 11010802027423号
京公网安备 11010802027423号