当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Regio- and stereoselective synthesis of 1,4-enynes by iron-catalysed Suzuki-Miyaura coupling of propargyl electrophiles under ligand-free conditions.
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-04-29 , DOI: 10.1039/d0ob00357c Ryosuke Agata 1 , Siming Lu 1 , Hiroshi Matsuda 2 , Katsuhiro Isozaki 1 , Masaharu Nakamura 1
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-04-29 , DOI: 10.1039/d0ob00357c Ryosuke Agata 1 , Siming Lu 1 , Hiroshi Matsuda 2 , Katsuhiro Isozaki 1 , Masaharu Nakamura 1
Affiliation
|
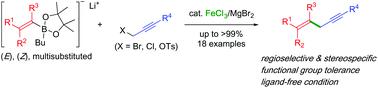
The first iron-catalysed cross coupling of propargyl electrophiles with lithium alkenylborates has been developed. Various propargyl electrophiles can be cross-coupled with lithium (E)- or (Z)-alkenylborates in a stereospecific manner to afford the corresponding 1,4-enynes in good to excellent yields. The reaction features high SN2-type regioselectivity and functional group compatibility.
中文翻译:

在无配体条件下,通过铁催化的炔丙基亲电试剂的Suzuki-Miyaura偶联进行区域和立体选择性合成1,4-炔烃。
已经开发了炔丙基亲电试剂与链烯基硼酸锂的第一铁催化的交叉偶联。各种炔丙基亲电试剂可以立体定向的方式与(E)-或(Z)-烯基硼酸锂交联,以良好至极好的收率得到相应的1,4-烯炔。该反应具有高的SN2型区域选择性和官能团相容性。
更新日期:2020-03-25
中文翻译:

在无配体条件下,通过铁催化的炔丙基亲电试剂的Suzuki-Miyaura偶联进行区域和立体选择性合成1,4-炔烃。
已经开发了炔丙基亲电试剂与链烯基硼酸锂的第一铁催化的交叉偶联。各种炔丙基亲电试剂可以立体定向的方式与(E)-或(Z)-烯基硼酸锂交联,以良好至极好的收率得到相应的1,4-烯炔。该反应具有高的SN2型区域选择性和官能团相容性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号