当前位置:
X-MOL 学术
›
Org. Biomol. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Organocatalytic asymmetric addition of thioglycolates to o-quinone methides: a route to 5-substituted-5H-benzoxathiepine-2(3H)-ones.
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-04-15 , DOI: 10.1039/d0ob00568a Chandan Gharui 1 , Satya Prakash 2 , Deepak Chopra 2 , Subhas Chandra Pan 1
Organic & Biomolecular Chemistry ( IF 2.9 ) Pub Date : 2020-04-15 , DOI: 10.1039/d0ob00568a Chandan Gharui 1 , Satya Prakash 2 , Deepak Chopra 2 , Subhas Chandra Pan 1
Affiliation
|
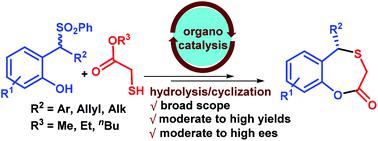
Herein we have developed the first enantioselective synthesis of 5-substituted-5H-benzoxathiepine-2(3H)-ones. 2-Sulfonylmethyl phenols and thioglycolates were employed as reaction partners in this method. The desired thia Michael products were obtained via bifunctional squaramide catalyzed conjugate addition reaction to in situ generated o-quinone methides and then basic hydrolysis followed by cyclization led to the formation of 5-substituted-5H-benzoxathiepine-2(3H)-ones. Broad scope and moderate to high enantioselectivities were observed for both products.
中文翻译:

硫代乙醇酸酯的有机催化不对称加成至邻醌甲基化物:通往5取代的5H-苯并噻吩-2(3H)-的途径。
在本文中,我们开发了5-取代的-5H-苯并噻吩-2-2(3H)-的第一个对映选择性合成。在该方法中,使用2-磺酰基甲基苯酚和巯基乙酸盐作为反应伙伴。通过双官能方酰胺催化的共轭加成反应,原位生成邻醌甲基化物,然后进行碱性水解,然后环化,导致形成5-取代的-5H-苯并噻吩-2(3H)-一,得到所需的迈克尔产物。两种产品均观察到宽范围和中等至高对映选择性。
更新日期:2020-04-24
中文翻译:

硫代乙醇酸酯的有机催化不对称加成至邻醌甲基化物:通往5取代的5H-苯并噻吩-2(3H)-的途径。
在本文中,我们开发了5-取代的-5H-苯并噻吩-2-2(3H)-的第一个对映选择性合成。在该方法中,使用2-磺酰基甲基苯酚和巯基乙酸盐作为反应伙伴。通过双官能方酰胺催化的共轭加成反应,原位生成邻醌甲基化物,然后进行碱性水解,然后环化,导致形成5-取代的-5H-苯并噻吩-2(3H)-一,得到所需的迈克尔产物。两种产品均观察到宽范围和中等至高对映选择性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号