当前位置:
X-MOL 学术
›
Chem. Soc. Rev.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Halogen bonds of halonium ions.
Chemical Society Reviews ( IF 40.4 ) Pub Date : 2020-05-11 , DOI: 10.1039/d0cs00034e Lotta Turunen 1 , Máté Erdélyi 1
Chemical Society Reviews ( IF 40.4 ) Pub Date : 2020-05-11 , DOI: 10.1039/d0cs00034e Lotta Turunen 1 , Máté Erdélyi 1
Affiliation
|
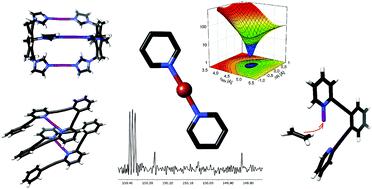
Due to their electron deficiency, halonium ions act as particularly strong halogen bond donors. By accepting electrons in both lobes of their empty p-orbital, they are capable of simultaneously interacting with two Lewis bases. The interaction presumes the formation of three molecular orbitals and is accordingly typically entitled as a three-center halogen bond. In analogy to the [D-H-D]+ hydrogen bonds, which are at times entitled as short and strong bonds, the [D-X-D]+ halogen bonds of halonium ions show Bondi normalized interatomic distances of 0.6-0.7 and possess both charge transfer and electrostatic characteristics. The three-center halogen bond of halonium ions shows distinct differences in its properties from coordinative bonds of transition metals and is therefore applicable as a complementary synthon in supramolecular chemistry. The three-center halogen bond modulates the reactivity of halonium ions and is hence a useful tool for synthetic organic chemistry. Following the discussion of the nature and properties of halonium ions' halogen bonds, this tutorial review provides an overview of their current applications to stimulate future developments.
中文翻译:

lon离子的卤素键。
由于缺乏电子,ha离子可作为特别强的卤素键供体。通过接受空p轨道的两个叶中的电子,它们能够同时与两个Lewis碱基相互作用。相互作用假定形成三个分子轨道,因此通常被称为三中心卤素键。类似于[DHD] +氢键(有时称为短键和强键),ha离子的[DXD] +卤素键显示邦迪标准化原子间距为0.6-0.7,并具有电荷转移和静电特性。lon离子的三中心卤素键与过渡金属的配位键在性质上有明显不同,因此可作为超分子化学中的互补合成子使用。三中心卤素键调节ha离子的反应性,因此是合成有机化学的有用工具。在讨论了离子卤素键的性质和特性之后,本教程回顾概述了their离子当前的应用以刺激未来的发展。
更新日期:2020-03-25
中文翻译:

lon离子的卤素键。
由于缺乏电子,ha离子可作为特别强的卤素键供体。通过接受空p轨道的两个叶中的电子,它们能够同时与两个Lewis碱基相互作用。相互作用假定形成三个分子轨道,因此通常被称为三中心卤素键。类似于[DHD] +氢键(有时称为短键和强键),ha离子的[DXD] +卤素键显示邦迪标准化原子间距为0.6-0.7,并具有电荷转移和静电特性。lon离子的三中心卤素键与过渡金属的配位键在性质上有明显不同,因此可作为超分子化学中的互补合成子使用。三中心卤素键调节ha离子的反应性,因此是合成有机化学的有用工具。在讨论了离子卤素键的性质和特性之后,本教程回顾概述了their离子当前的应用以刺激未来的发展。











































 京公网安备 11010802027423号
京公网安备 11010802027423号