PLoS Pathogens ( IF 5.5 ) Pub Date : 2020-03-25 , DOI: 10.1371/journal.ppat.1008435 Laís Amorim Sacramento 1 , Luciana Benevides 1, 2 , Sandra Regina Maruyama 3 , Lucas Tavares 4 , Kiyoshi Ferreira Fukutani 1 , Marcela Francozo 5 , Tim Sparwasser 5, 6 , Fernando Queiroz Cunha 7 , Roque Pacheco Almeida 8 , João Santana da Silva 1, 2 , Vanessa Carregaro 1
|
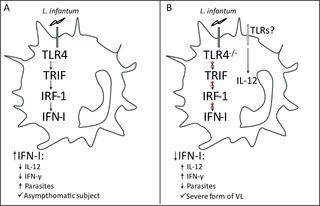
A striking feature of human visceral leishmaniasis (VL) is chronic inflammation in the spleen and liver, and VL patients present increased production levels of multiple inflammatory mediators, which contribute to tissue damage and disease severity. Here, we combined an experimental model with the transcriptional profile of human VL to demonstrate that the TLR4-IFN-β pathway regulates the chronic inflammatory process and is associated with the asymptomatic form of the disease. Tlr4-deficient mice harbored fewer parasites in their spleen and liver than wild-type mice. TLR4 deficiency enhanced the Th1 immune response against the parasite, which was correlated with an increased activation of dendritic cells (DCs). Gene expression analyses demonstrated that IRF1 and IFN-β were expressed downstream of TLR4 after infection. Accordingly, IRF1- and IFNAR-deficient mice harbored fewer parasites in the target organs than wild-type mice due to having an increased Th1 immune response. However, the absence of TLR4 or IFNAR increased the serum transaminase levels in infected mice, indicating the presence of liver damage in these animals. In addition, IFN-β limits IFN-γ production by acting directly on Th1 cells. Using RNA sequencing analysis of human samples, we demonstrated that the transcriptional signature for the TLR4 and type I IFN (IFN-I) pathways was positively modulated in asymptomatic subjects compared with VL patients and thus provide direct evidence demonstrating that the TLR4-IFN-I pathway is related to the nondevelopment of the disease. In conclusion, our results demonstrate that the TLR4-IRF1 pathway culminates in IFN-β production as a mechanism for dampening the chronic inflammatory process and preventing immunopathology development.
中文翻译:

TLR4通过IRF1和IFN-β消除Th1免疫应答,以防止婴儿乳杆菌感染期间的免疫病理变化。
人内脏利什曼病(VL)的一个显着特征是脾脏和肝脏的慢性炎症,并且VL患者表现出多种炎症介质增加的产生水平,这导致组织损伤和疾病严重程度。在这里,我们将实验模型与人类VL的转录谱相结合,以证明TLR4-IFN-β途径调节慢性炎症过程,并与疾病的无症状形式相关。Tlr4缺陷小鼠的脾脏和肝脏中的寄生虫数量少于野生型小鼠。TLR4缺乏症增强了针对寄生虫的Th1免疫应答,这与树突状细胞(DC)的激活增加有关。基因表达分析表明感染后IRF1和IFN-β在TLR4下游表达。因此,由于具有增加的Th1免疫应答,IRF1和IFNAR缺陷型小鼠在靶器官中的寄生虫少于野生型小鼠。但是,没有TLR4或IFNAR会增加感染小鼠的血清转氨酶水平,表明这些动物存在肝损害。此外,IFN-β通过直接作用于Th1细胞来限制IFN-γ的产生。使用人类样品的RNA测序分析,我们证明,与VL患者相比,无症状受试者中TLR4和I型IFN(IFN-I)途径的转录签名受到正向调节,因此提供了直接的证据证明TLR4-IFN-I途径与TLR4-IFN-I的未发育有关。疾病。总之,我们的结果表明,TLR4-IRF1途径最终导致IFN-β的产生,从而抑制了慢性炎症过程并阻止了免疫病理学发展。











































 京公网安备 11010802027423号
京公网安备 11010802027423号