Communications Chemistry ( IF 5.9 ) Pub Date : 2020-03-25 , DOI: 10.1038/s42004-020-0284-3 Bobo Xing 1 , Nigel Graham 2 , Wenzheng Yu 1, 2
|
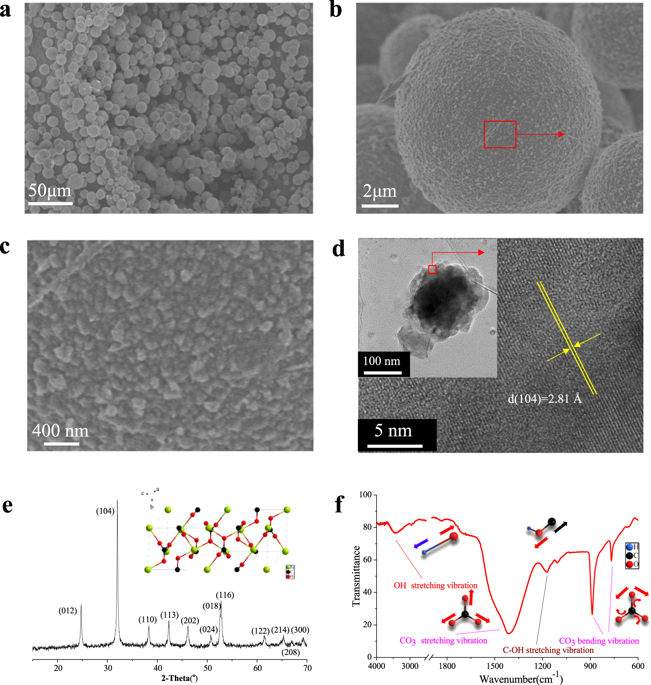
Humic acid (HA) is particularly important in iron-bearing mineral transformations and erosion at the water-mineral boundary zone of the Earth. In this study, three stages of the possible pathway by which HA causes mineral transformation from siderite to goethite are identified. Firstly, a Fe(II)-HA complex is formed by chelation, which accelerates the dissolution and oxidation of Fe(II) from the surface of siderite. As the Fe(II)-HA complex retains Fe atoms in close proximity of each other, ferrihydrite is formed by the agglomeration and crystallization. Finally, the ferrihydrite structurally rearranges upon attachment to the surface of goethite crystals and merges with its structure. The influence of low concentrations of HA (0–2 mg/L) on phosphate adsorption is found to be beneficial by the inducing of new mineral phases. We believe that these results provide a greater understanding of the impact of HA in the biogeochemical cycle of phosphate, mineral transformation.
中文翻译:

自然环境中腐殖酸将菱铁矿转化为针铁矿
腐植酸(HA)对于地球水-矿物边界带的含铁矿物转化和侵蚀特别重要。在这项研究中,确定了 HA 导致矿物从菱铁矿转变为针铁矿的可能途径的三个阶段。首先,螯合形成Fe(II)-HA络合物,加速Fe(II)从菱铁矿表面的溶解和氧化。由于 Fe(II)-HA 络合物使 Fe 原子彼此靠近,因此通过团聚和结晶形成水铁矿。最后,水铁矿在附着到针铁矿晶体表面后结构重新排列并与其结构融合。低浓度 HA (0–2 mg/L) 对磷酸盐吸附的影响被发现有利于诱导新的矿物相。我们相信这些结果可以更好地了解 HA 在磷酸盐、矿物转化的生物地球化学循环中的影响。











































 京公网安备 11010802027423号
京公网安备 11010802027423号