Nature ( IF 50.5 ) Pub Date : 2020-03-25 , DOI: 10.1038/s41586-020-2136-9 Fabian M Arnold 1 , Miriam S Weber 2 , Imre Gonda 1 , Marc J Gallenito 3 , Sophia Adenau 1 , Pascal Egloff 1, 4 , Iwan Zimmermann 1, 4 , Cedric A J Hutter 1 , Lea M Hürlimann 1 , Eike E Peters 5 , Jörn Piel 5 , Gabriele Meloni 3 , Ohad Medalia 2 , Markus A Seeger 1
|
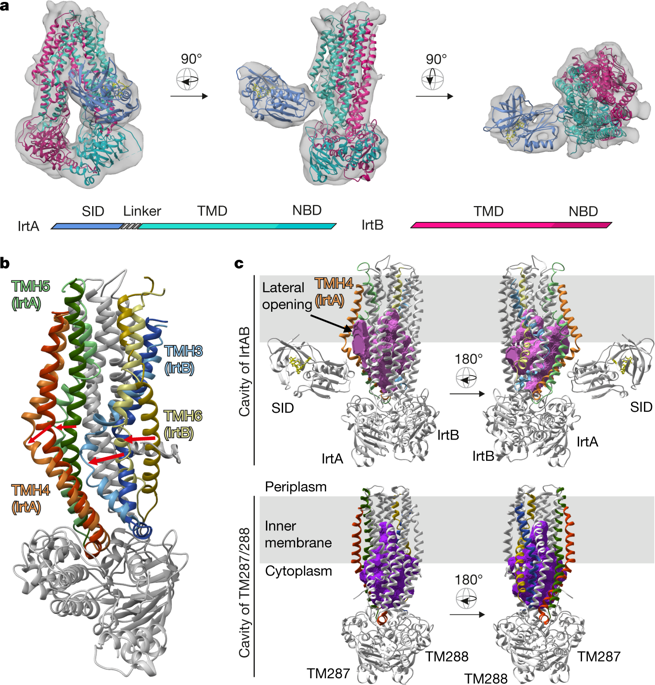
Intracellular replication of the deadly pathogen Mycobacterium tuberculosis relies on the production of small organic molecules called siderophores that scavenge iron from host proteins1. M. tuberculosis produces two classes of siderophore, lipid-bound mycobactin and water-soluble carboxymycobactin2,3. Functional studies have revealed that iron-loaded carboxymycobactin is imported into the cytoplasm by the ATP binding cassette (ABC) transporter IrtAB4, which features an additional cytoplasmic siderophore interaction domain5. However, the predicted ABC exporter fold of IrtAB is seemingly contradictory to its import function. Here we show that membrane-reconstituted IrtAB is sufficient to import mycobactins, which are then reduced by the siderophore interaction domain to facilitate iron release. Structure determination by X-ray crystallography and cryo-electron microscopy not only confirms that IrtAB has an ABC exporter fold, but also reveals structural peculiarities at the transmembrane region of IrtAB that result in a partially collapsed inward-facing substrate-binding cavity. The siderophore interaction domain is positioned in close proximity to the inner membrane leaflet, enabling the reduction of membrane-inserted mycobactin. Enzymatic ATPase activity and in vivo growth assays show that IrtAB has a preference for mycobactin over carboxymycobactin as its substrate. Our study provides insights into an unusual ABC exporter that evolved as highly specialized siderophore-import machinery in mycobacteria.
中文翻译:

ABC 出口商 IrtAB 进口并减少分枝杆菌铁载体
致命病原体结核分枝杆菌的细胞内复制依赖于产生称为铁载体的有机小分子,这些小分子可从宿主蛋白中清除铁1。结核分枝杆菌产生两类铁载体,脂质结合的分枝杆菌素和水溶性羧基分枝杆菌素2,3。功能研究表明,负载铁的羧基霉菌素通过 ATP 结合盒 (ABC) 转运蛋白 IrtAB 4输入细胞质,该转运蛋白具有额外的细胞质铁载体相互作用结构域5. 然而,预测的 IrtAB 的 ABC 出口折叠似乎与其进口功能相矛盾。在这里,我们表明膜重组的 IrtAB 足以导入分枝杆菌素,然后通过铁载体相互作用域减少分枝杆菌素以促进铁释放。通过 X 射线晶体学和低温电子显微镜确定结构不仅证实了 IrtAB 具有 ABC 输出折叠,而且还揭示了 IrtAB 跨膜区域的结构特性,导致部分塌陷的向内底物结合腔。铁载体相互作用域位于靠近内膜小叶的位置,从而能够减少膜插入的分枝杆菌素。酶促 ATP 酶活性和体内生长试验表明,IrtAB 对作为底物的分枝杆菌素优于羧基分枝杆菌素。











































 京公网安备 11010802027423号
京公网安备 11010802027423号