Nature ( IF 50.5 ) Pub Date : 2020-03-25 , DOI: 10.1038/s41586-020-2134-y Barbara Maier 1, 2, 3 , Andrew M Leader 1, 2, 3 , Steven T Chen 1, 2, 3 , Navpreet Tung 1, 2 , Christie Chang 1, 4 , Jessica LeBerichel 1, 2, 3 , Aleksey Chudnovskiy 1, 2, 3, 5 , Shrisha Maskey 1, 2, 3 , Laura Walker 1, 4 , John P Finnigan 1, 2 , Margaret E Kirkling 6, 7 , Boris Reizis 6 , Sourav Ghosh 8 , Natalie Roy D'Amore 9 , Nina Bhardwaj 1, 2, 10, 11 , Carla V Rothlin 12 , Andrea Wolf 13 , Raja Flores 13 , Thomas Marron 1, 2, 10 , Adeeb H Rahman 1, 4, 14 , Ephraim Kenigsberg 1, 14, 15 , Brian D Brown 1, 2, 14 , Miriam Merad 1, 2, 3, 4
|
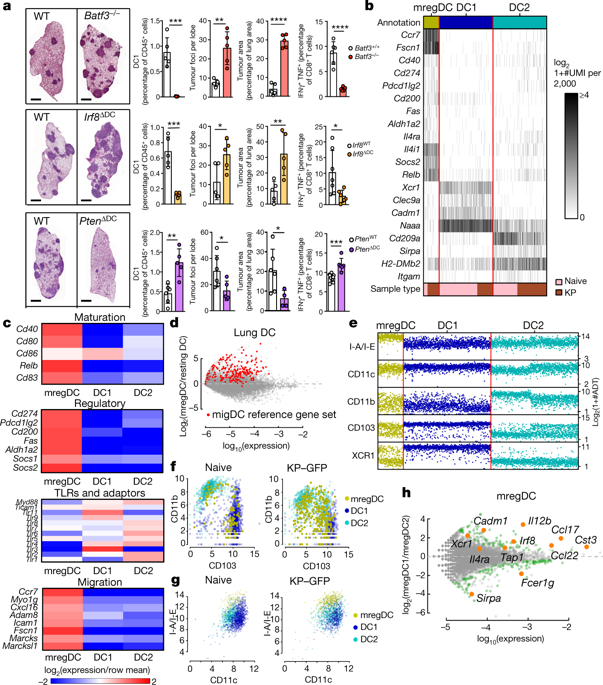
Checkpoint blockade therapies have improved cancer treatment, but such immunotherapy regimens fail in a large subset of patients. Conventional type 1 dendritic cells (DC1s) control the response to checkpoint blockade in preclinical models and are associated with better overall survival in patients with cancer, reflecting the specialized ability of these cells to prime the responses of CD8+ T cells1,2,3. Paradoxically, however, DC1s can be found in tumours that resist checkpoint blockade, suggesting that the functions of these cells may be altered in some lesions. Here, using single-cell RNA sequencing in human and mouse non-small-cell lung cancers, we identify a cluster of dendritic cells (DCs) that we name ‘mature DCs enriched in immunoregulatory molecules’ (mregDCs), owing to their coexpression of immunoregulatory genes (Cd274, Pdcd1lg2 and Cd200) and maturation genes (Cd40, Ccr7 and Il12b). We find that the mregDC program is expressed by canonical DC1s and DC2s upon uptake of tumour antigens. We further find that upregulation of the programmed death ligand 1 protein—a key checkpoint molecule—in mregDCs is induced by the receptor tyrosine kinase AXL, while upregulation of interleukin (IL)-12 depends strictly on interferon-γ and is controlled negatively by IL-4 signalling. Blocking IL-4 enhances IL-12 production by tumour-antigen-bearing mregDC1s, expands the pool of tumour-infiltrating effector T cells and reduces tumour burden. We have therefore uncovered a regulatory module associated with tumour-antigen uptake that reduces DC1 functionality in human and mouse cancers.
中文翻译:

保守的树突状细胞调节程序限制了抗肿瘤免疫
检查点阻断疗法改善了癌症治疗,但这种免疫治疗方案在很大一部分患者中失败。常规 1 型树突状细胞 (DC1) 在临床前模型中控制对检查点阻断的反应,并与癌症患者更好的总生存期相关,这反映了这些细胞启动 CD8 + T 细胞反应的特化能力1,2,3. 然而,矛盾的是,DC1s 可以在抵抗检查点阻断的肿瘤中发现,这表明这些细胞的功能可能在某些病变中发生改变。在这里,我们在人类和小鼠非小细胞肺癌中使用单细胞 RNA 测序,鉴定出一组树突状细胞 (DC),我们将其命名为“富含免疫调节分子的成熟 DC”(mregDC),因为它们的共表达免疫调节基因 ( Cd274 , Pdcd1lg2和Cd200 ) 和成熟基因 ( C d40 , C cr7和Il12b ))。我们发现 mregDC 程序在摄取肿瘤抗原后由典型的 DC1s 和 DC2s 表达。我们进一步发现,mregDCs 中程序性死亡配体 1 蛋白(一种关键的检查点分子)的上调是由受体酪氨酸激酶 AXL 诱导的,而白细胞介素 (IL)-12 的上调严格依赖于干扰素-γ,并受 IL 负调控-4 信号。阻断 IL-4 可增强携带肿瘤抗原的 mregDC1s 产生 IL-12,扩大肿瘤浸润效应 T 细胞库并减少肿瘤负荷。因此,我们发现了一个与肿瘤抗原摄取相关的调节模块,它降低了人类和小鼠癌症中 DC1 的功能。











































 京公网安备 11010802027423号
京公网安备 11010802027423号