Journal of Molecular Liquids ( IF 5.3 ) Pub Date : 2020-03-24 , DOI: 10.1016/j.molliq.2020.112973 Claudia Maria Simonescu , Vasile Lavric , Ancuta Musina , Oana Maria Antonescu , Daniela Cristina Culita , Virgil Marinescu , Christu Tardei , Ovidiu Oprea , Andreea Madalina Pandele
|
Nowadays, the concerns regarding cadmium pollution increased steadily, due to its serious damages to the environment and human health. Consequently, stringent limits have been set for this heavy metal ion both in industrial and drinking water. Numerous traditional and unconventional methods were applied to reach these limits.
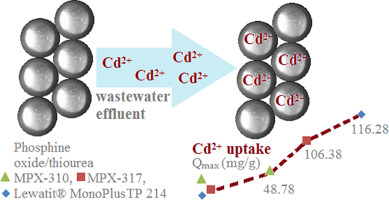
In this paper, two phosphine oxide/phosphine oxide and thiourea chelating resins MPX-310 and MPX-317 produced by Magpie Polymers - Italmatch Chemicals, Italy were comparatively evaluated with a commercial thiourea chelating resin Lewatit® MonoPlus TP 214 produced by Lanxess regarding Cd(II) removal from aqueous synthetic solutions. The physicochemical properties of the resins were studied by FTIR spectroscopy, scanning electron microscopy (SEM), thermogravimetric analysis (TG-DSC) and X-ray Photoelectron Spectroscopy (XPS).
Batch tests have been performed to assess the performance of these resins in Cd(II) removal. A value of 3–4 for pH and 600 min of contact time were determined as optimal parameters for the removal process. The experimental results also showed that the Lewatit® MonoPlus TP 214 has a higher removal capacity than MPX-317 and MPX-310 due to the presence of the thiourea and ion-exchange functional groups on the Lewatit® MonoPlus TP 214 backbone, compared with the presence of the phosphine oxide functional groups on the MPX-310 and thiourea and phosphine oxide on MPX-317. A monolayer adsorption on the homogenous adsorbent surface mechanism involved in Cd(II) removal was pointed out by the results regarding adsorption isotherms. The best fit of the pseudo-second kinetic model indicates that the sorption process goes through a mechanism that involves chemical reaction between Cd(II) and functional groups grafted on the resin's backbone. The chelating capacity of the tested resins showed that they are suitable for Cd(II) removal from aqueous synthetic solutions and industrial wastewater.
中文翻译:

螯合树脂去除镉离子的实验与建模
如今,由于镉污染严重损害环境和人类健康,对镉污染的关注稳步增加。因此,对工业用水和饮用水中的重金属离子都设定了严格的限值。许多传统的和非传统的方法都被用来达到这些极限。
本文对意大利Magpie Polymers-Italmatch Chemicals生产的两种氧化膦/氧化膦和硫脲螯合树脂MPX-310和MPX-317与商用朗盛生产的硫脲螯合树脂Lewatit®MonoPlus TP 214进行了Cd( II)从水性合成溶液中去除。通过FTIR光谱,扫描电子显微镜(SEM),热重分析(TG-DSC)和X射线光电子能谱(XPS)研究了树脂的理化性质。
已经进行了分批测试,以评估这些树脂在Cd(II)去除中的性能。确定pH值为3-4,接触时间为600分钟是去除过程的最佳参数。实验结果还表明,由于Lewatit®MonoPlus TP 214骨架上存在硫脲和离子交换官能团,因此Lewatit®MonoPlus TP 214的去除能力高于MPX-317和MPX-310。在MPX-310上存在氧化膦官能团,在MPX-317上存在硫脲和氧化膦。有关吸附等温线的结果指出了在吸附Cd(II)的均质吸附剂表面机理上的单层吸附。拟秒动力学模型的最佳拟合表明吸附过程经历了一种机制,该机制涉及Cd(II)与接枝在树脂骨架上的官能团之间的化学反应。测试树脂的螯合能力表明它们适合从合成水溶液和工业废水中去除Cd(II)。











































 京公网安备 11010802027423号
京公网安备 11010802027423号