当前位置:
X-MOL 学术
›
Appl. Surf. Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Understanding the role of boron and stoichiometric ratio in the catalytic performance of amorphous Co-B catalyst
Applied Surface Science ( IF 6.3 ) Pub Date : 2020-07-01 , DOI: 10.1016/j.apsusc.2020.146199 R. Kadrekar , N. Patel , A. Arya
Applied Surface Science ( IF 6.3 ) Pub Date : 2020-07-01 , DOI: 10.1016/j.apsusc.2020.146199 R. Kadrekar , N. Patel , A. Arya
|
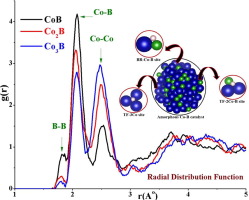
Abstract The structural, electronic and hydrogen adsorption properties of amorphous CoB, Co2B and Co3B structures were investigated using first principle calculations to evaluate their catalytic activity for HER. A total of eight different sites were considered for hydrogen adsorption, of which the threefold site with two Co and single B atom (TF-2Co-B) was found to be most active site for all the three amorphous Co-B structures. The B atom donates electronic charge to the Co-B bond, while, the presence of Co atom is responsible for achieving the optimal charge density necessary for favorable hydrogen adsorption. This interaction between the Co and B atoms makes bonding region between the Co and B atoms most favorable for hydrogen adsorption and not lone Co atom nor the B atom, thus, determining the active sites of Co-B catalyst. The best catalytic behavior of Co2B stoichiometry was credited to the perfect balance of magnetic properties, optimum hydrogen adsorption energies and considerable number of active sites it displayed.
中文翻译:

了解硼和化学计量比在无定形 Co-B 催化剂催化性能中的作用
摘要 使用第一性原理计算研究了无定形 CoB、Co2B 和 Co3B 结构的结构、电子和氢吸附性能,以评估它们对 HER 的催化活性。总共考虑了八个不同的氢吸附位点,其中发现具有两个 Co 和单个 B 原子的三重位点(TF-2Co-B)是所有三种无定形 Co-B 结构中最活跃的位点。B 原子为 Co-B 键提供电荷,而 Co 原子的存在负责实现有利的氢吸附所需的最佳电荷密度。Co 和 B 原子之间的这种相互作用使 Co 和 B 原子之间的键合区域最有利于氢吸附,而不是孤立的 Co 原子和 B 原子,从而决定了 Co-B 催化剂的活性位点。
更新日期:2020-07-01
中文翻译:

了解硼和化学计量比在无定形 Co-B 催化剂催化性能中的作用
摘要 使用第一性原理计算研究了无定形 CoB、Co2B 和 Co3B 结构的结构、电子和氢吸附性能,以评估它们对 HER 的催化活性。总共考虑了八个不同的氢吸附位点,其中发现具有两个 Co 和单个 B 原子的三重位点(TF-2Co-B)是所有三种无定形 Co-B 结构中最活跃的位点。B 原子为 Co-B 键提供电荷,而 Co 原子的存在负责实现有利的氢吸附所需的最佳电荷密度。Co 和 B 原子之间的这种相互作用使 Co 和 B 原子之间的键合区域最有利于氢吸附,而不是孤立的 Co 原子和 B 原子,从而决定了 Co-B 催化剂的活性位点。











































 京公网安备 11010802027423号
京公网安备 11010802027423号