当前位置:
X-MOL 学术
›
Mater. Today
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Activation and stabilization mechanisms of anionic redox for Li storage applications: Joint experimental and theoretical study on Li2TiO3–LiMnO2 binary system
Materials Today ( IF 21.1 ) Pub Date : 2020-07-01 , DOI: 10.1016/j.mattod.2020.03.002 Yuki Kobayashi , Miho Sawamura , Sayaka Kondo , Maho Harada , Yusuke Noda , Masanobu Nakayama , Sho Kobayakawa , Wenwen Zhao , Aiko Nakao , Akira Yasui , Hongahally Basappa Rajendra , Keisuke Yamanaka , Toshiaki Ohta , Naoaki Yabuuchi
Materials Today ( IF 21.1 ) Pub Date : 2020-07-01 , DOI: 10.1016/j.mattod.2020.03.002 Yuki Kobayashi , Miho Sawamura , Sayaka Kondo , Maho Harada , Yusuke Noda , Masanobu Nakayama , Sho Kobayakawa , Wenwen Zhao , Aiko Nakao , Akira Yasui , Hongahally Basappa Rajendra , Keisuke Yamanaka , Toshiaki Ohta , Naoaki Yabuuchi
|
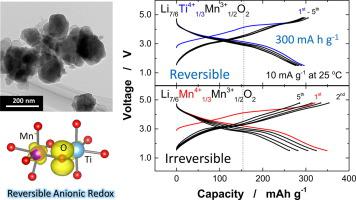
Abstract A binary system of Li2TiO3–LiMnO2 is systematically examined by joint experimental and theoretical studies as electrode materials for Li storage applications. Increase in a fraction of Li2TiO3 effectively activates anionic redox, and thus holes are reversibly formed on oxygen by electrochemical oxidation. Such holes are energetically stabilized through π-type interaction with Mn t2g orbital as suggested by theoretical calculation. However, excess enrichment of Li2TiO3 fractions in this binary system results in the oxygen loss as an irreversible process on delithiation because of a non-bonding character for Ti–O bonds coupled with the formation of O–O dimers, which are chemically and electrochemically unstable species. Additionally, detailed electrochemical study clearly shows that Li migration kinetics is relatively slow, presumably coupled with low electronic conductivity. Nevertheless, nanosizing of primary particles is an effective strategy to overcome this limitation. The nanosized sample prepared by mechanical milling delivers a large reversible capacity, ∼300 mA h g−1, even at room temperature and shows much improved capacity retention. Formation and stabilization of holes for the nanosized sample are also directly evidenced by soft X-ray absorption spectroscopy. From these results, factors affecting the reversibility of anionic redox as emerging new chemistry and its possibility for energy storage applications are discussed in more details.
中文翻译:

锂存储应用中阴离子氧化还原的活化和稳定机制:Li2TiO3-LiMnO2二元体系的联合实验和理论研究
摘要 通过联合实验和理论研究,系统地研究了 Li2TiO3-LiMnO2 二元体系作为锂存储应用的电极材料。增加一部分 Li2TiO3 有效地激活阴离子氧化还原,因此通过电化学氧化在氧上可逆地形成空穴。如理论计算所示,这些空穴通过与 Mn t2g 轨道的 π 型相互作用在能量上稳定。然而,由于 Ti-O 键的非键合特性以及化学和电化学不稳定的 O-O 二聚体的形成,在该二元体系中过量富集 Li2TiO3 会导致脱锂过程中不可逆的氧损失物种。此外,详细的电化学研究清楚地表明锂迁移动力学相对较慢,可能与低电子电导率有关。然而,初级粒子的纳米尺寸是克服这一限制的有效策略。通过机械研磨制备的纳米级样品即使在室温下也能提供大的可逆容量,~300 mAh g-1,并显示出大大提高的容量保持率。软 X 射线吸收光谱也直接证明了纳米尺寸样品孔的形成和稳定。从这些结果中,更详细地讨论了影响阴离子氧化还原可逆性的因素作为新兴的新化学及其在储能应用中的可能性。通过机械研磨制备的纳米级样品即使在室温下也能提供大的可逆容量,~300 mAh g-1,并显示出大大提高的容量保持率。软 X 射线吸收光谱也直接证明了纳米尺寸样品孔的形成和稳定。从这些结果中,更详细地讨论了影响阴离子氧化还原可逆性的因素作为新兴的新化学及其在储能应用中的可能性。通过机械研磨制备的纳米级样品即使在室温下也能提供大的可逆容量,~300 mAh g-1,并显示出大大提高的容量保持率。软 X 射线吸收光谱也直接证明了纳米尺寸样品孔的形成和稳定。从这些结果中,更详细地讨论了影响阴离子氧化还原可逆性的因素作为新兴的新化学及其在储能应用中的可能性。
更新日期:2020-07-01
中文翻译:

锂存储应用中阴离子氧化还原的活化和稳定机制:Li2TiO3-LiMnO2二元体系的联合实验和理论研究
摘要 通过联合实验和理论研究,系统地研究了 Li2TiO3-LiMnO2 二元体系作为锂存储应用的电极材料。增加一部分 Li2TiO3 有效地激活阴离子氧化还原,因此通过电化学氧化在氧上可逆地形成空穴。如理论计算所示,这些空穴通过与 Mn t2g 轨道的 π 型相互作用在能量上稳定。然而,由于 Ti-O 键的非键合特性以及化学和电化学不稳定的 O-O 二聚体的形成,在该二元体系中过量富集 Li2TiO3 会导致脱锂过程中不可逆的氧损失物种。此外,详细的电化学研究清楚地表明锂迁移动力学相对较慢,可能与低电子电导率有关。然而,初级粒子的纳米尺寸是克服这一限制的有效策略。通过机械研磨制备的纳米级样品即使在室温下也能提供大的可逆容量,~300 mAh g-1,并显示出大大提高的容量保持率。软 X 射线吸收光谱也直接证明了纳米尺寸样品孔的形成和稳定。从这些结果中,更详细地讨论了影响阴离子氧化还原可逆性的因素作为新兴的新化学及其在储能应用中的可能性。通过机械研磨制备的纳米级样品即使在室温下也能提供大的可逆容量,~300 mAh g-1,并显示出大大提高的容量保持率。软 X 射线吸收光谱也直接证明了纳米尺寸样品孔的形成和稳定。从这些结果中,更详细地讨论了影响阴离子氧化还原可逆性的因素作为新兴的新化学及其在储能应用中的可能性。通过机械研磨制备的纳米级样品即使在室温下也能提供大的可逆容量,~300 mAh g-1,并显示出大大提高的容量保持率。软 X 射线吸收光谱也直接证明了纳米尺寸样品孔的形成和稳定。从这些结果中,更详细地讨论了影响阴离子氧化还原可逆性的因素作为新兴的新化学及其在储能应用中的可能性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号