当前位置:
X-MOL 学术
›
Water Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Superfast degradation of refractory organic contaminants by ozone activated with thiosulfate: Efficiency and mechanisms.
Water Research ( IF 11.4 ) Pub Date : 2020-03-25 , DOI: 10.1016/j.watres.2020.115751 Jingxin Yang 1 , Cheng Luo 2 , Tingting Li 3 , Jie Cao 3 , Wenyi Dong 3 , Ji Li 3 , Jun Ma 4
Water Research ( IF 11.4 ) Pub Date : 2020-03-25 , DOI: 10.1016/j.watres.2020.115751 Jingxin Yang 1 , Cheng Luo 2 , Tingting Li 3 , Jie Cao 3 , Wenyi Dong 3 , Ji Li 3 , Jun Ma 4
Affiliation
|
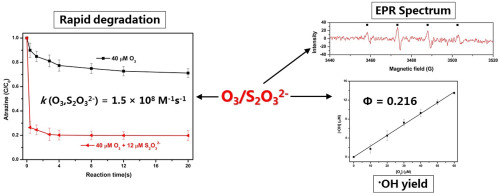
Thiosulfate (S2O32-) is frequently used as an ozone (O3) quenching agent when investigating the ozonation of organic contaminants and the kinetics thereof. In this study, however, O3 is activated by S2O32-, resulting in a superfast degradation of O3-refractory contaminants. Therefore, the focus of this study is the exploration into the enhancing role of S2O32- in the degradation of refractory organic contaminants by O3, which has been overlooked thus far. Results obtained from scavenging experiments and electron paramagnetic resonance (EPR) spectra verify that •OH generated from the reaction of S2O32- with O3 is mainly responsible for the superfast degradation of O3-refractory contaminants. The •OH yield from the O3/S2O32- process is determined to be 0.216. A plausible mechanism for the generation of •OH from the O3/S2O32- process is proposed with the implementation of density functional theory (DFT). Initially, ozone reacts with a sulfur of S2O32- to form OOOSSO32-. The adduct then rearranges to OO(O)SSO32- or HOO(O)SSO32- in the presence of H+, which cleaves to give a sulfoxide radical cation and O2•-/HO2•. O2•-/HO2• is rapidly transformed into •OH by O3 through a series of steps. Degradation efficiency of O3-refractory contaminants of this process highly depends on the molar ratio of S2O32- and O3 ([S2O32-]:[O3]). The optimal [S2O32-]:[O3] is pH dependent in synthetic water (e.g. 0.3 at pH 7). The presence of bicarbonate inhibits the degradation of refractory contaminants by the O3/S2O32- process. Humic acid exhibits a slight enhancing effect at low concentrations (0.1-0.2 mg-C/L), and an inhibiting effect at higher concentrations (≥0.4 mg-C/L). In addition, the efficacy of the O3/S2O32- process in real water matrices is also confirmed.
中文翻译:

硫代硫酸盐活化的臭氧可快速降解难处理的有机污染物:效率和机理。
在研究有机污染物的臭氧化及其动力学时,硫代硫酸盐(S2O32-)通常用作臭氧(O3)淬灭剂。但是,在这项研究中,O3被S2O32-活化,导致O3难降解污染物超快降解。因此,本研究的重点是探索S2O32-在O3降解难降解有机污染物中的增强作用,这一点迄今被人们所忽视。通过清除实验和电子顺磁共振(EPR)光谱获得的结果证明,由S2O32-与O3反应生成的•OH主要负责O3难降解污染物的超快降解。由O3 / S2O32-过程获得的•OH产率确定为0.216。通过实施密度泛函理论(DFT),提出了一种由O3 / S2O32-过程生成•OH的合理机制。最初,臭氧与S2O32-的硫反应生成OOOSSO32-。然后,在H +存在下,加合物重排为OO(O)SSO32-或HOO(O)SSO32-,它们裂解生成亚砜自由基阳离子和O2•-/ HO2•。O2•-/ HO2•通过一系列步骤被O3迅速转化为•OH。此过程中O3难熔污染物的降解效率高度取决于S2O32-和O3的摩尔比([S2O32-]:[O3])。最佳的[S2O32-]:[O3]与合成水中的pH有关(例如,pH为7时为0.3)。碳酸氢盐的存在抑制了O3 / S2O32-工艺对难处理污染物的降解。在低浓度(0.1-0.2 mg-C / L)下,腐殖酸表现出轻微的增强作用,在较高浓度(≥0.4mg-C / L)下具有抑制作用。此外,还证实了O3 / S2O32-方法在真实水基质中的功效。
更新日期:2020-03-26
中文翻译:

硫代硫酸盐活化的臭氧可快速降解难处理的有机污染物:效率和机理。
在研究有机污染物的臭氧化及其动力学时,硫代硫酸盐(S2O32-)通常用作臭氧(O3)淬灭剂。但是,在这项研究中,O3被S2O32-活化,导致O3难降解污染物超快降解。因此,本研究的重点是探索S2O32-在O3降解难降解有机污染物中的增强作用,这一点迄今被人们所忽视。通过清除实验和电子顺磁共振(EPR)光谱获得的结果证明,由S2O32-与O3反应生成的•OH主要负责O3难降解污染物的超快降解。由O3 / S2O32-过程获得的•OH产率确定为0.216。通过实施密度泛函理论(DFT),提出了一种由O3 / S2O32-过程生成•OH的合理机制。最初,臭氧与S2O32-的硫反应生成OOOSSO32-。然后,在H +存在下,加合物重排为OO(O)SSO32-或HOO(O)SSO32-,它们裂解生成亚砜自由基阳离子和O2•-/ HO2•。O2•-/ HO2•通过一系列步骤被O3迅速转化为•OH。此过程中O3难熔污染物的降解效率高度取决于S2O32-和O3的摩尔比([S2O32-]:[O3])。最佳的[S2O32-]:[O3]与合成水中的pH有关(例如,pH为7时为0.3)。碳酸氢盐的存在抑制了O3 / S2O32-工艺对难处理污染物的降解。在低浓度(0.1-0.2 mg-C / L)下,腐殖酸表现出轻微的增强作用,在较高浓度(≥0.4mg-C / L)下具有抑制作用。此外,还证实了O3 / S2O32-方法在真实水基质中的功效。











































 京公网安备 11010802027423号
京公网安备 11010802027423号