当前位置:
X-MOL 学术
›
J. Colloid Interface Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Probing the hydrophobic mechanism of N-[(3-hydroxyamino)-propoxy]-N-octyl dithiocarbamate toward bastnaesite flotation by in situ AFM, FTIR and XPS.
Journal of Colloid and Interface Science ( IF 9.4 ) Pub Date : 2020-03-23 , DOI: 10.1016/j.jcis.2020.03.080 Jing Qi 1 , Guangyi Liu 1 , Yan Dong 1
Journal of Colloid and Interface Science ( IF 9.4 ) Pub Date : 2020-03-23 , DOI: 10.1016/j.jcis.2020.03.080 Jing Qi 1 , Guangyi Liu 1 , Yan Dong 1
Affiliation
|
HYPOTHESIS
Both hydroxamate and dithiocarbamate groups exhibit a unique bonding characteristic toward rare earth ions. A hydroxamic acid surfactant containing a dithiocarbamate group should possess a specific affinity to hydrophobize bastnaesite [(Ce, La)CO3F] flotation.
EXPERIMENTS
N-[(3-hydroxyamino)-propoxy]-N-octyl dithiocarbamate (OAHD) was synthesized, and its flotation mechanism toward bastnaesite was investigated by in situ AFM, FTIR, XPS, micro-flotation and contact angle.
FINDINGS
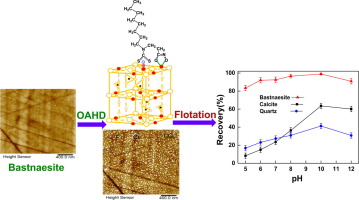
In situ AFM clearly observed that OAHD aggregated on bastnaesite surface, which improved the contact angle and surface hydrophobicity of bastnaesite. FTIR spectra and XPS recommended that OAHD's dithiocarbamate and hydroxamate groups co-anchored on bastnaesite surface through strong chemisorption, which strengthened the bonding affinity of bastnaesite toward OAHD. UV spectra showed that both dithiocarbamate and hydroxamate groups exhibited weak affinity toward Ca2+ ions, which benefited OAHD's selective flotation separation of bastnaesite from calcite. The co-adsorption and special hydrophobic structure improved OAHD's flotation performance. As a result, OAHD returned higher flotation selectivity for bastnaesite than OHA (n-octyl hydroxamic acid) which chemisorbed on bastnaesite surface only through the hydroxamate group and used the heptyl as hydrophobic group.
中文翻译:

通过原位AFM,FTIR和XPS探讨了N-[(3-羟氨基)-丙氧基] -N-辛基二硫代氨基甲酸酯对马氏体浮选的疏水机理。
假设异羟肟酸酯基团和二硫代氨基甲酸酯基团均表现出对稀土离子的独特键合特性。含有二硫代氨基甲酸酯基团的异羟肟酸表面活性剂应具有特定的亲和力,以疏水化玄武岩[(Ce,La)CO3F]浮选。实验合成了N-[(3-羟基氨基)-丙氧基] -N-辛基二硫代氨基甲酸酯(OAHD),并通过原位原子力显微镜,傅立叶变换红外光谱(FTIR),XPS,微浮选和接触角研究了其对镁橄榄石的浮选机理。结果原位原子力显微镜清楚地观察到,OAHD聚集在镁橄榄石表面,从而改善了镁橄榄石的接触角和表面疏水性。FTIR光谱和XPS建议OAHD的二硫代氨基甲酸酯和异羟肟酸酯基团通过强化学吸附作用共锚固在镁铝石表面上,这增强了贝氏体对OAHD的键合亲和力。紫外光谱表明,二硫代氨基甲酸酯基和异羟肟酸酯基团均对Ca2 +离子表现出弱亲和力,这有利于OAHD从方解石中选择性地浮选分离出的玄武岩。共吸附和特殊的疏水结构提高了OAHD的浮选性能。结果,与仅通过异羟肟酸酯基团化学吸附在镁铝石表面上并使用庚基作为疏水基团的OHA(正辛基异羟肟酸)相比,OAHD对镁铝石的浮选选择性更高。浮选性能。结果,与仅通过异羟肟酸酯基团化学吸附在镁铝石表面上并使用庚基作为疏水基团的OHA(正辛基异羟肟酸)相比,OAHD对镁铝石的浮选选择性更高。浮选性能。结果,与仅通过异羟肟酸酯基团化学吸附在镁铝石表面上并使用庚基作为疏水基团的OHA(正辛基异羟肟酸)相比,OAHD对镁铝石的浮选选择性更高。
更新日期:2020-03-26
中文翻译:

通过原位AFM,FTIR和XPS探讨了N-[(3-羟氨基)-丙氧基] -N-辛基二硫代氨基甲酸酯对马氏体浮选的疏水机理。
假设异羟肟酸酯基团和二硫代氨基甲酸酯基团均表现出对稀土离子的独特键合特性。含有二硫代氨基甲酸酯基团的异羟肟酸表面活性剂应具有特定的亲和力,以疏水化玄武岩[(Ce,La)CO3F]浮选。实验合成了N-[(3-羟基氨基)-丙氧基] -N-辛基二硫代氨基甲酸酯(OAHD),并通过原位原子力显微镜,傅立叶变换红外光谱(FTIR),XPS,微浮选和接触角研究了其对镁橄榄石的浮选机理。结果原位原子力显微镜清楚地观察到,OAHD聚集在镁橄榄石表面,从而改善了镁橄榄石的接触角和表面疏水性。FTIR光谱和XPS建议OAHD的二硫代氨基甲酸酯和异羟肟酸酯基团通过强化学吸附作用共锚固在镁铝石表面上,这增强了贝氏体对OAHD的键合亲和力。紫外光谱表明,二硫代氨基甲酸酯基和异羟肟酸酯基团均对Ca2 +离子表现出弱亲和力,这有利于OAHD从方解石中选择性地浮选分离出的玄武岩。共吸附和特殊的疏水结构提高了OAHD的浮选性能。结果,与仅通过异羟肟酸酯基团化学吸附在镁铝石表面上并使用庚基作为疏水基团的OHA(正辛基异羟肟酸)相比,OAHD对镁铝石的浮选选择性更高。浮选性能。结果,与仅通过异羟肟酸酯基团化学吸附在镁铝石表面上并使用庚基作为疏水基团的OHA(正辛基异羟肟酸)相比,OAHD对镁铝石的浮选选择性更高。浮选性能。结果,与仅通过异羟肟酸酯基团化学吸附在镁铝石表面上并使用庚基作为疏水基团的OHA(正辛基异羟肟酸)相比,OAHD对镁铝石的浮选选择性更高。









































 京公网安备 11010802027423号
京公网安备 11010802027423号