当前位置:
X-MOL 学术
›
Solid State Sci.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis by solution combustion of inorganic blue pigments MFe2(P2O7)2: Influence of the cation M (Zn2+, Co2+, Cu2+, and Mg2+) on their optical properties
Solid State Sciences ( IF 3.4 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.solidstatesciences.2020.106180 E.A. Chavarriaga , A.A. Lopera , T.B. Wermuth , S. Arcaro , A. Gómez , O.J. Restrepo , J. Alarcón , C.P. Bergmann
Solid State Sciences ( IF 3.4 ) Pub Date : 2020-05-01 , DOI: 10.1016/j.solidstatesciences.2020.106180 E.A. Chavarriaga , A.A. Lopera , T.B. Wermuth , S. Arcaro , A. Gómez , O.J. Restrepo , J. Alarcón , C.P. Bergmann
|
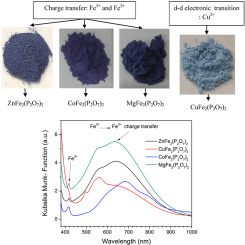
Abstract The aim of this work is to find the effect of the cation M (Zn2+, Co2+, Cu2+, and Mg2+) on the optical properties of MFe2(P2O7)2 synthesized by solution combustion. The crystal structure of the powders was determined by X-ray diffraction (XRD), Infrared spectroscopy (IR), and Raman spectroscopy. The presence of Fe2+ and Fe3+ ions in the pigments was studied using Mossbauer spectroscopy (MS), these spectra showed at 298 K three well-defined Mossbauer doublets belonging to octahedral S1 site and S3 site of Fe3+ and trigonal prismatic S2 site of Fe2+, confirming the formation of ZnFe2(P2O7)2, CoFe2(P2O7)2 and MgFe2(P2O7)2 structures, while in CuFe2(P2O7)2 there is no presence of trigonal prismatic S2 site of Fe2+ ion. Therefore, the mechanism of color on the ZnFe2(P2O7)2, CoFe2(P2O7)2, and MgFe2(P2O7)2 structures are due to intervalence charge transfer between Fe2+ and Fe3+ ions. In contrast, on the CuFe2(P2O7)2 structure is due to d-d electronic transitions of the Cu2+ ion, these evidences can also be observed in their UV–Vis diffuse reflectance spectra. Moreover, the inorganic blue pigment MgFe2(P2O7)2, which is free of cobalt, copper, and zinc could be used as a pigment in applications of low temperatures.
中文翻译:

通过溶液燃烧合成无机蓝色颜料 MFe2(P2O7)2:阳离子 M(Zn2+、Co2+、Cu2+ 和 Mg2+)对其光学性质的影响
摘要 本工作的目的是寻找阳离子 M(Zn2+、Co2+、Cu2+ 和 Mg2+)对溶液燃烧合成 MFe2(P2O7)2 光学性质的影响。通过X射线衍射(XRD)、红外光谱(IR)和拉曼光谱确定粉末的晶体结构。使用穆斯堡尔光谱 (MS) 研究了颜料中 Fe2+ 和 Fe3+ 离子的存在,这些光谱在 298 K 显示三个明确的穆斯堡尔双峰,属于 Fe3+ 的八面体 S1 位点和 S3 位点以及 Fe2+ 的三棱柱 S2 位点,证实ZnFe2(P2O7)2、CoFe2(P2O7)2 和 MgFe2(P2O7)2 结构的形成,而在 CuFe2(P2O7)2 中不存在 Fe2+ 离子的三角棱柱 S2 位点。因此,ZnFe2(P2O7)2、CoFe2(P2O7)2、和 MgFe2(P2O7)2 结构是由于 Fe2+ 和 Fe3+ 离子之间的间隔电荷转移。相反,在 CuFe2(P2O7)2 结构上是由于 Cu2+ 离子的 dd 电子跃迁,这些证据也可以在它们的 UV-Vis 漫反射光谱中观察到。此外,不含钴、铜和锌的无机蓝色颜料 MgFe2(P2O7)2 可用作低温应用中的颜料。
更新日期:2020-05-01
中文翻译:

通过溶液燃烧合成无机蓝色颜料 MFe2(P2O7)2:阳离子 M(Zn2+、Co2+、Cu2+ 和 Mg2+)对其光学性质的影响
摘要 本工作的目的是寻找阳离子 M(Zn2+、Co2+、Cu2+ 和 Mg2+)对溶液燃烧合成 MFe2(P2O7)2 光学性质的影响。通过X射线衍射(XRD)、红外光谱(IR)和拉曼光谱确定粉末的晶体结构。使用穆斯堡尔光谱 (MS) 研究了颜料中 Fe2+ 和 Fe3+ 离子的存在,这些光谱在 298 K 显示三个明确的穆斯堡尔双峰,属于 Fe3+ 的八面体 S1 位点和 S3 位点以及 Fe2+ 的三棱柱 S2 位点,证实ZnFe2(P2O7)2、CoFe2(P2O7)2 和 MgFe2(P2O7)2 结构的形成,而在 CuFe2(P2O7)2 中不存在 Fe2+ 离子的三角棱柱 S2 位点。因此,ZnFe2(P2O7)2、CoFe2(P2O7)2、和 MgFe2(P2O7)2 结构是由于 Fe2+ 和 Fe3+ 离子之间的间隔电荷转移。相反,在 CuFe2(P2O7)2 结构上是由于 Cu2+ 离子的 dd 电子跃迁,这些证据也可以在它们的 UV-Vis 漫反射光谱中观察到。此外,不含钴、铜和锌的无机蓝色颜料 MgFe2(P2O7)2 可用作低温应用中的颜料。











































 京公网安备 11010802027423号
京公网安备 11010802027423号