当前位置:
X-MOL 学术
›
BBA Biomembr.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
31P and 13C solid-state NMR analysis of morphological changes of phospholipid bilayers containing glucagon during fibril formation of glucagon under neutral condition.
Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 2.8 ) Pub Date : 2020-03-25 , DOI: 10.1016/j.bbamem.2020.183290 Kazumi Haya 1 , Yoshiteru Makino 1 , Akie Kikuchi-Kinoshita 1 , Izuru Kawamura 1 , Akira Naito 1
Biochimica et Biophysica Acta (BBA) - Biomembranes ( IF 2.8 ) Pub Date : 2020-03-25 , DOI: 10.1016/j.bbamem.2020.183290 Kazumi Haya 1 , Yoshiteru Makino 1 , Akie Kikuchi-Kinoshita 1 , Izuru Kawamura 1 , Akira Naito 1
Affiliation
|
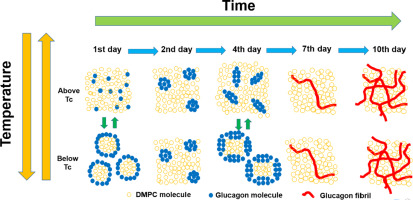
Glucagon is a 29 amino acid peptide hormone secreted by pancreatic α-cells that interacts with specific receptors located in various organs. Glucagon tends to form gel-like fibrillar aggregates that are cytotoxic due to their activation of apoptotic signaling pathways. To understand the glucagon-membrane interactions, morphological changes in dimyristoylphosphatidylcholine (DMPC) bilayers containing glucagon in neutral solution were investigated by observing 31P NMR spectra. First, lipid bilayers with a DMPC/glucagon molar ratio of 50/1 were observed. One day after preparing the DMPC/glucagon lipid bilayer sample, lipid bilayers were disrupted below the phase transition temperature (Tc). Membrane disruption was reduced 2 days after preparation due to the reduction of glucagon-DMPC interaction, and subsequently increased by 4 days and was reduced again by 7 days. TEM measurements showed that small ellipsoidal intermediates of glucagon were observed inside the small size of lipid bilayer after 4 days, while fibrils grew inside lipid bilayer after 19 days. These results indicate that morphological changes in DMPC/glucagon lipid bilayers are correlated with the evolution of glucagon aggregate state. Particularly, fibril intermediate shows a strong glucagon lipid bilayer interaction. We further investigated the structure and kinetics of glucagon fibril formation inside the DMPC lipid bilayer in a neutral solution using 13C solid-state NMR spectroscopy. α-Helical structures were observed around Gly4 and Ala19 in the monomeric form, which changed to β-sheet structures in the fibril form. The fibrillation process can be explained by a two-step autocatalytic reaction mechanism in which the first step is a homogeneous nuclear formation (k1), and the second step is an autocatalytic heterogeneous fibrillation process (k2).
中文翻译:

31 P和13 C固态NMR分析中性条件下胰高血糖素原纤维形成过程中含胰高血糖素的磷脂双层的形态变化。
胰高血糖素是由胰腺α细胞分泌的29个氨基酸的肽激素,与位于各个器官的特定受体相互作用。胰高血糖素趋于形成由于细胞凋亡信号通路的活化而具有细胞毒性的凝胶状原纤维聚集体。为了了解胰高血糖素-膜的相互作用,通过观察31 P NMR光谱研究了中性溶液中含有胰高血糖素的二豆油基磷脂酰胆碱(DMPC)双层的形态变化。首先,观察到DMPC /胰高血糖素摩尔比为50/1的脂质双层。在制备DMPC /胰高血糖素脂质双层样品的一天后,在相变温度(Tc)以下破坏脂质双层。由于胰高血糖素-DMPC相互作用的减少,制备后2天的膜破坏减少,然后增加了4天,又减少了7天。TEM测量显示,在4天后在小的脂质双层中观察到了胰高血糖素的小椭圆形中间体,而在19天后在脂质双层内生长了原纤维。这些结果表明,DMPC /胰高血糖素脂质双层中的形态变化与胰高血糖素聚集状态的演变相关。特别地,原纤维中间体显示强胰高血糖素脂质双层相互作用。我们进一步使用13 C固态NMR光谱研究了中性溶液中DMPC脂质双层内部胰高血糖素原纤维形成的结构和动力学。在单体形式的Gly4和Ala19周围观察到α-螺旋结构,其变为原纤维形式的β-折叠结构。
更新日期:2020-03-26
中文翻译:

31 P和13 C固态NMR分析中性条件下胰高血糖素原纤维形成过程中含胰高血糖素的磷脂双层的形态变化。
胰高血糖素是由胰腺α细胞分泌的29个氨基酸的肽激素,与位于各个器官的特定受体相互作用。胰高血糖素趋于形成由于细胞凋亡信号通路的活化而具有细胞毒性的凝胶状原纤维聚集体。为了了解胰高血糖素-膜的相互作用,通过观察31 P NMR光谱研究了中性溶液中含有胰高血糖素的二豆油基磷脂酰胆碱(DMPC)双层的形态变化。首先,观察到DMPC /胰高血糖素摩尔比为50/1的脂质双层。在制备DMPC /胰高血糖素脂质双层样品的一天后,在相变温度(Tc)以下破坏脂质双层。由于胰高血糖素-DMPC相互作用的减少,制备后2天的膜破坏减少,然后增加了4天,又减少了7天。TEM测量显示,在4天后在小的脂质双层中观察到了胰高血糖素的小椭圆形中间体,而在19天后在脂质双层内生长了原纤维。这些结果表明,DMPC /胰高血糖素脂质双层中的形态变化与胰高血糖素聚集状态的演变相关。特别地,原纤维中间体显示强胰高血糖素脂质双层相互作用。我们进一步使用13 C固态NMR光谱研究了中性溶液中DMPC脂质双层内部胰高血糖素原纤维形成的结构和动力学。在单体形式的Gly4和Ala19周围观察到α-螺旋结构,其变为原纤维形式的β-折叠结构。









































 京公网安备 11010802027423号
京公网安备 11010802027423号