Cell Reports ( IF 8.8 ) Pub Date : 2020-03-24 , DOI: 10.1016/j.celrep.2020.03.003 Heng Zhang , Maëva Devoucoux , Xiaosheng Song , Li Li , Gamze Ayaz , Harry Cheng , Wolfram Tempel , Cheng Dong , Peter Loppnau , Jacques Côté , Jinrong Min
|
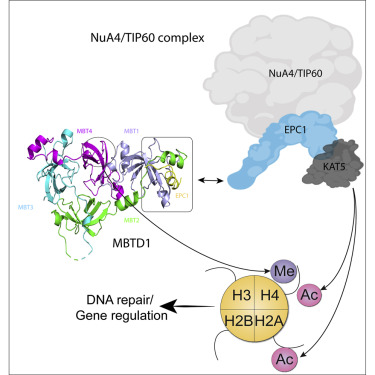
MBTD1, a H4K20me reader, has recently been identified as a component of the NuA4/TIP60 acetyltransferase complex, regulating gene expression and DNA repair. NuA4/TIP60 inhibits 53BP1 binding to chromatin through recognition of the H4K20me mark by MBTD1 and acetylation of H2AK15, blocking the ubiquitination mark required for 53BP1 localization at DNA breaks. The NuA4/TIP60 non-catalytic subunit EPC1 enlists MBTD1 into the complex, but the detailed molecular mechanism remains incompletely explored. Here, we present the crystal structure of the MBTD1-EPC1 complex, revealing a hydrophobic C-terminal fragment of EPC1 engaging the MBT repeats of MBTD1 in a site distinct from the H4K20me binding site. Different cellular assays validate the physiological significance of the key residues involved in the MBTD1-EPC1 interaction. Our study provides a structural framework for understanding the mechanism by which MBTD1 recruits the NuA4/TIP60 acetyltransferase complex to influence transcription and DNA repair pathway choice.
中文翻译:

EPC1介导的MBTD1进入NuA4 / TIP60乙酰转移酶复合物的结构基础
MBTD1,一种H4K20me读卡器,最近已被确定为NuA4 / TIP60乙酰转移酶复合物的组成部分,可调节基因表达和DNA修复。NuA4 / TIP60通过MBTD1和H2AK15的乙酰化识别H4K20me标志,从而抑制53BP1与染色质的结合,从而阻断了53BP1在DNA断裂处定位所需的泛素化标志。NuA4 / TIP60非催化亚基EPC1使MBTD1进入该复合物中,但详细的分子机理仍未完全探索。在这里,我们介绍了MBTD1-EPC1复合物的晶体结构,揭示了EPC1的疏水性C端片段与MBTD1的MBT重复序列结合在不同于H4K20me结合位点的位点。不同的细胞测定法验证了MBTD1-EPC1相互作用中涉及的关键残基的生理学意义。



























 京公网安备 11010802027423号
京公网安备 11010802027423号