Nano Energy ( IF 16.8 ) Pub Date : 2020-03-25 , DOI: 10.1016/j.nanoen.2020.104727 Biao Li , Yifeng Wang , Ning Jiang , Li An , Jin Song , Yuxuan Zuo , Fanghua Ning , Huaifang Shang , Dingguo Xia
|
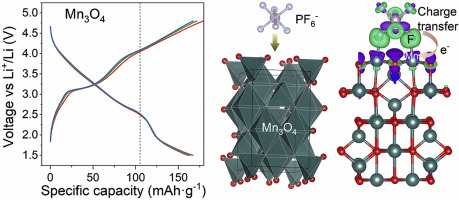
Conventional batteries (except redox flow batteries) react by involving only their electrode redox centers, with electrolyte ions shuttled between the cathode and the anode. However, this chemistry causes large electronic and structural changes in the bulk of the electrode materials, leading to performance degradation. Herein, we report that electrolytic-anion-redox adsorption pseudocapacitance, relayed by lithium intercalation in lithium-free transition metal oxide electrodes, can be exploited to boost the total energy density of a battery. Focused on the study of Mn3O4, we show that this pseudocapacitance is processed by the reversible adsorption/desorption of PF6− on the surface of transition metal oxides, with charge transfer between Mn3O4 and PF6− through Mn–F bond, with fluoride ions acting as redox centers. This new electrolytic-anion-redox chemistry provides greater design freedom for high-capacity energy storage devices since it only involves a specific electrode surface and the electrolyte anion, rather than specially crystallized compounds for bulk reactions.
中文翻译:

纳米无锂过渡金属氧化物作为锂离子电池正极材料中的电解-阴离子-氧化还原吸附假电容
常规电池(氧化还原液流电池除外)通过仅涉及其电极氧化还原中心进行反应,电解质离子在阴极和阳极之间穿梭。但是,这种化学反应会导致大量电极材料发生较大的电子和结构变化,从而导致性能下降。在本文中,我们报道了可以利用锂嵌入无锂过渡金属氧化物电极中的电解质阴离子-氧化还原吸附假电容来提高电池的总能量密度。集中在Mn的研究3 Ò 4,我们表明,这种赝由PF的可逆吸附/解吸处理6 -过渡金属氧化物的表面上,与锰之间的电荷转移3 Ò 4和PF 6 -至Mn-F键,与氟离子作为氧化还原中心。这种新的电解-阴离子-氧化还原化学方法为大容量储能设备提供了更大的设计自由度,因为它仅涉及特定的电极表面和电解质阴离子,而不是用于本体反应的专门结晶的化合物。











































 京公网安备 11010802027423号
京公网安备 11010802027423号