当前位置:
X-MOL 学术
›
Organometallics
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Synthesis and Reactivity of PtII Methyl Complexes Supported by Pyrazolate Pincer Ligands
Organometallics ( IF 2.5 ) Pub Date : 2020-03-25 , DOI: 10.1021/acs.organomet.0c00023 Braden A. Zahora 1, 2 , Michael R. Gau 1 , Karen I. Goldberg 1
Organometallics ( IF 2.5 ) Pub Date : 2020-03-25 , DOI: 10.1021/acs.organomet.0c00023 Braden A. Zahora 1, 2 , Michael R. Gau 1 , Karen I. Goldberg 1
Affiliation
|
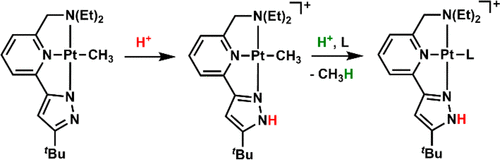
Multidentate pyrazolate ligands, 2-(5-tert-butylpyrazol-3-yl)-6-(diethylaminomethyl)pyridine (HNNN)Et and 2,6-bis(5-tert-butyl-1H-pyrazol-3-yl)pyridine (HNNNH), were metalated with PtII to form neutral Pt(*NNN)EtCH3 and anionic [Y][Pt(*NNN*)CH3] (Y = Li(THF)−Cl−Li(THF)3; Y = PPN) (* denotes that the pyrazolate N has been deprotonated). Reactions of these PtII–CH3 complexes with acids were investigated. Protonation occurs preferentially at the pyrazolate site, and the release of methane requires additional acid.
中文翻译:

吡唑啉酸盐夹杂配体负载的Pt II甲基配合物的合成与反应性
多齿吡唑酸酯配体,2-(5-叔丁基吡唑-3-基)-6-(二乙基氨基甲基)吡啶(H NNN)Et和2,6-双(5-叔丁基-1 H-吡唑-3-基)吡啶(H NNN H),用Pt II金属化,形成中性Pt(* NNN)Et CH 3和阴离子[Y] [Pt(* NNN *)CH 3 ](Y = Li(THF)-Cl-Li (THF)3; Y = PPN)(*表示吡唑酸酯N已去质子化)。这些Pt II –CH 3的反应研究了与酸的配合物。质子化优先发生在吡唑酸酯位置,甲烷的释放需要额外的酸。
更新日期:2020-03-25
中文翻译:

吡唑啉酸盐夹杂配体负载的Pt II甲基配合物的合成与反应性
多齿吡唑酸酯配体,2-(5-叔丁基吡唑-3-基)-6-(二乙基氨基甲基)吡啶(H NNN)Et和2,6-双(5-叔丁基-1 H-吡唑-3-基)吡啶(H NNN H),用Pt II金属化,形成中性Pt(* NNN)Et CH 3和阴离子[Y] [Pt(* NNN *)CH 3 ](Y = Li(THF)-Cl-Li (THF)3; Y = PPN)(*表示吡唑酸酯N已去质子化)。这些Pt II –CH 3的反应研究了与酸的配合物。质子化优先发生在吡唑酸酯位置,甲烷的释放需要额外的酸。











































 京公网安备 11010802027423号
京公网安备 11010802027423号