当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
A Binary Arginine Methylation Switch on Histone H3 Arginine 2 Regulates Its Interaction with WDR5.
Biochemistry ( IF 2.9 ) Pub Date : 2020-03-24 , DOI: 10.1021/acs.biochem.0c00035 Benjamin M Lorton 1 , Rajesh K Harijan 1 , Emmanuel S Burgos 1 , Jeffrey B Bonanno 1 , Steven C Almo 1 , David Shechter 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-03-24 , DOI: 10.1021/acs.biochem.0c00035 Benjamin M Lorton 1 , Rajesh K Harijan 1 , Emmanuel S Burgos 1 , Jeffrey B Bonanno 1 , Steven C Almo 1 , David Shechter 1
Affiliation
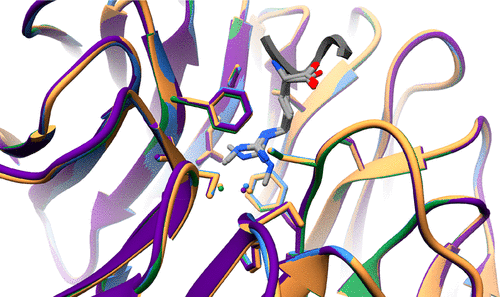
|
Histone H3 arginine 2 (H3R2) is post-translationally modified in three different states by “writers” of the protein arginine methyltransferase (PRMT) family. H3R2 methylarginine isoforms include PRMT5-catalyzed monomethylation (me1) and symmetric dimethylation (me2s) and PRMT6-catalyzed me1 and asymmetric dimethylation (me2a). WD-40 repeat-containing protein 5 (WDR5) is an epigenetic “reader” protein that interacts with H3R2. Previous studies suggested that H3R2me2s specified a high-affinity interaction with WDR5. However, our prior biological data prompted the hypothesis that WDR5 may also interact with H3R2me1. Here, using highly accurate quantitative binding analysis combined with high-resolution crystal structures of WDR5 in complex with unmodified (me0) and me1/me2s l-arginine amino acids and in complex with the H3R2me1 peptide, we provide a rigorous biochemical study and address long-standing discrepancies of this important biological interaction. Despite modest structural differences at the binding interface, our study supports an interaction model regulated by a binary arginine methylation switch: H3R2me2a prevents interaction with WDR5, whereas H3R2me0, -me1, and -me2s are equally permissive.
中文翻译:

组蛋白 H3 精氨酸 2 上的二元精氨酸甲基化开关调节其与 WDR5 的相互作用。
组蛋白 H3 精氨酸 2 (H3R2) 被蛋白质精氨酸甲基转移酶 (PRMT) 家族的“编写者”以三种不同的状态进行翻译后修饰。H3R2 甲基精氨酸异构体包括 PRMT5 催化的单甲基化 (me1) 和对称二甲基化 (me2s) 以及 PRMT6 催化的 me1 和不对称二甲基化 (me2a)。含有 WD-40 重复序列的蛋白 5 (WDR5) 是一种与 H3R2 相互作用的表观遗传“阅读器”蛋白。以前的研究表明 H3R2me2s 指定了与 WDR5 的高亲和力相互作用。然而,我们之前的生物学数据提示了 WDR5 也可能与 H3R2me1 相互作用的假设。在这里,使用高度准确的定量结合分析结合 WDR5 的高分辨率晶体结构与未修饰的 (me0) 和 me1/me2s l复合-精氨酸氨基酸并与 H3R2me1 肽复合,我们提供了严格的生化研究并解决了这种重要生物相互作用的长期差异。尽管结合界面存在适度的结构差异,但我们的研究支持由二元精氨酸甲基化开关调节的相互作用模型:H3R2me2a 阻止与 WDR5 的相互作用,而 H3R2me0、-me1 和 -me2s 则同样允许。
更新日期:2020-03-24
中文翻译:

组蛋白 H3 精氨酸 2 上的二元精氨酸甲基化开关调节其与 WDR5 的相互作用。
组蛋白 H3 精氨酸 2 (H3R2) 被蛋白质精氨酸甲基转移酶 (PRMT) 家族的“编写者”以三种不同的状态进行翻译后修饰。H3R2 甲基精氨酸异构体包括 PRMT5 催化的单甲基化 (me1) 和对称二甲基化 (me2s) 以及 PRMT6 催化的 me1 和不对称二甲基化 (me2a)。含有 WD-40 重复序列的蛋白 5 (WDR5) 是一种与 H3R2 相互作用的表观遗传“阅读器”蛋白。以前的研究表明 H3R2me2s 指定了与 WDR5 的高亲和力相互作用。然而,我们之前的生物学数据提示了 WDR5 也可能与 H3R2me1 相互作用的假设。在这里,使用高度准确的定量结合分析结合 WDR5 的高分辨率晶体结构与未修饰的 (me0) 和 me1/me2s l复合-精氨酸氨基酸并与 H3R2me1 肽复合,我们提供了严格的生化研究并解决了这种重要生物相互作用的长期差异。尽管结合界面存在适度的结构差异,但我们的研究支持由二元精氨酸甲基化开关调节的相互作用模型:H3R2me2a 阻止与 WDR5 的相互作用,而 H3R2me0、-me1 和 -me2s 则同样允许。











































 京公网安备 11010802027423号
京公网安备 11010802027423号