当前位置:
X-MOL 学术
›
Biochemistry
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Fluorescent Probes for Monitoring Serine Ubiquitination.
Biochemistry ( IF 2.9 ) Pub Date : 2020-03-27 , DOI: 10.1021/acs.biochem.0c00067 Kedar Puvar 1 , Aya M Saleh 2 , Ryan W Curtis 1 , Yiyang Zhou 1 , Prasanth R Nyalapatla 1 , Jiaqi Fu 3 , Alexander R Rovira 4 , Yitzhak Tor 4 , Zhao-Qing Luo 3 , Arun K Ghosh 1 , Mary J Wirth 1 , Jean Chmielewski 1 , Tamara L Kinzer-Ursem 2 , Chittaranjan Das 1
Biochemistry ( IF 2.9 ) Pub Date : 2020-03-27 , DOI: 10.1021/acs.biochem.0c00067 Kedar Puvar 1 , Aya M Saleh 2 , Ryan W Curtis 1 , Yiyang Zhou 1 , Prasanth R Nyalapatla 1 , Jiaqi Fu 3 , Alexander R Rovira 4 , Yitzhak Tor 4 , Zhao-Qing Luo 3 , Arun K Ghosh 1 , Mary J Wirth 1 , Jean Chmielewski 1 , Tamara L Kinzer-Ursem 2 , Chittaranjan Das 1
Affiliation
|
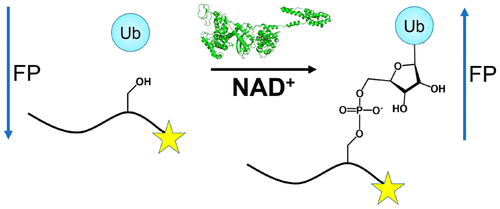
In a radical departure from the classical E1-E2-E3 three-enzyme mediated ubiquitination of eukaryotes, the recently described bacterial enzymes of the SidE family of Legionella pneumophila effectors utilize NAD+ to ligate ubiquitin onto target substrate proteins. This outcome is achieved via a two-step mechanism involving (1) ADP ribosylation of ubiquitin followed by (2) phosphotransfer to a target serine residue. Here, using fluorescent NAD+ analogues as well as synthetic substrate mimics, we have developed continuous assays enabling real-time monitoring of both steps of this mechanism. These assays are amenable to biochemical studies and high-throughput screening of inhibitors of these effectors, and the discovery and characterization of putative enzymes similar to members of the SidE family in other organisms. We also show their utility in studying enzymes that can reverse and inhibit this post-translational modification.
中文翻译:

用于监测丝氨酸泛素化的荧光探针。
与经典的E1-E2-E3三酶介导的真核生物泛素化有根本的不同,最近描述的嗜肺军团菌效应子SidE家族的细菌酶利用NAD +将泛素连接到靶底物蛋白上。该结果是通过两步机制实现的,该机制涉及(1)泛素的ADP核糖基化,然后(2)磷酸转移至靶标丝氨酸残基。在这里,我们使用荧光NAD +类似物以及合成的底物模拟物,开发出了连续检测方法,可以实时监控该机制的两个步骤。这些测定适用于这些效应子的抑制剂的生化研究和高通量筛选,以及与其他生物体中SidE家族成员相似的推定酶的发现和表征。
更新日期:2020-03-27
中文翻译:

用于监测丝氨酸泛素化的荧光探针。
与经典的E1-E2-E3三酶介导的真核生物泛素化有根本的不同,最近描述的嗜肺军团菌效应子SidE家族的细菌酶利用NAD +将泛素连接到靶底物蛋白上。该结果是通过两步机制实现的,该机制涉及(1)泛素的ADP核糖基化,然后(2)磷酸转移至靶标丝氨酸残基。在这里,我们使用荧光NAD +类似物以及合成的底物模拟物,开发出了连续检测方法,可以实时监控该机制的两个步骤。这些测定适用于这些效应子的抑制剂的生化研究和高通量筛选,以及与其他生物体中SidE家族成员相似的推定酶的发现和表征。











































 京公网安备 11010802027423号
京公网安备 11010802027423号