当前位置:
X-MOL 学术
›
Acc. Chem. Res.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrophilic Azides for Materials Synthesis and Chemical Biology.
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-03-24 , DOI: 10.1021/acs.accounts.0c00046 Sheng Xie 1 , Madanodaya Sundhoro 2 , K N Houk 3 , Mingdi Yan 2
Accounts of Chemical Research ( IF 16.4 ) Pub Date : 2020-03-24 , DOI: 10.1021/acs.accounts.0c00046 Sheng Xie 1 , Madanodaya Sundhoro 2 , K N Houk 3 , Mingdi Yan 2
Affiliation
|
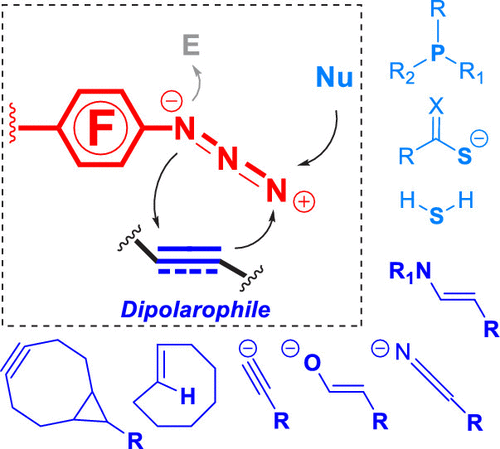
ConspectusOrganic azides are involved in a variety of useful transformations, including nitrene chemistry, reactions with nucleophiles and electrophiles, and cycloadditions. The 1,3-dipolar cycloadditions of azides constitute a major class of highly reliable and versatile reactions, as shown by the development and rapid adoption of click chemistry and bioorthogonal chemistry. Metal-catalyzed azide-alkyne cycloaddition (Cu/RuAAC), the prototypical click reaction, has found wide utility in pharmaceutical, biomedical, and materials sciences. The strain-promoted, or distortion-accelerated, azide-alkyne cycloaddition eliminates the need for a metal catalyst.In the azide-mediated 1,3-dipolar cycloaddition reactions, azides are ambiphilic, i.e., HOMO-LUMO-controlled dipoles where both the HOMO and LUMO interact strongly with the dipolarophile. Azide-alkyne cycloaddition proceeds primarily through the HOMOazide-LUMOdipolarophile interaction, and electron-deficient dipolarophiles react more readily. The inverse-electron-demand reaction, involving the LUMOazide-HOMOdipolarophile interaction, is less common because of the low stability of electron-deficient azides such as acyl, sulfonyl, and phosphoryl azides. Nevertheless, there have been reports since the 1960s showing enhanced reaction kinetics between electron-poor azides and electron-rich dipolarophiles. Our laboratory has developed the use of perfluoroaryl azides (PFAAs), a class of stable electron-deficient azides, as nitrene precursors and for reactions with nucleophiles and electron-rich dipolarophiles. Perfluorination on the aryl ring also facilitates the synthesis of PFAAs and quantitative analysis of the products by 19F NMR spectroscopy.In this Account, we summarize key reactions involving electrophilic azides and applications of these reactions in materials synthesis and chemical biology. These electron-deficient azides exhibit unique reactivity toward nucleophiles and electron-rich or strained dipolarophiles, in some cases leading to new transformations that do not require any catalysts or products that are impossible to obtain from the nonelectrophilic azides. We highlight work from our laboratories on reactions of PFAAs with enamines, enolates, thioacids, and phosphines. In the reactions of PFAAs with enamines or enolates, the triazole or triazoline cycloaddition products undergo further rearrangement to give amidines or amides as the final products at rates of up to 105 times faster than their non-fluorinated anlogues. Computational investigations by the distortion/interaction activation strain model reveal that perfluorination lowers the LUMO of the aryl azide as well as the overall activation energy of the reaction by decreasing the distortion energies of the reactants to reach the transition states. The PFAA-enamine reaction can be carried out in a one-pot fashion using readily available starting materials of aldehyde and amine, making the reaction especially attractive, for example, in the functionalization of nanomaterials and derivatization of antibiotics for the preparation of theranostic nanodrugs. Similar fast kinetics was also observed for the PPAA-mediated Staudinger reaction, which proceeds at 104 times higher rate than the classic Staudinger ligation, giving stable phosphoimines in high yields. The reaction is biorthogonal, allowing cell-surface labeling with minimal background noise.
中文翻译:

用于材料合成和化学生物学的亲电子叠氮化物。
概论有机叠氮化物参与多种有用的转化,包括腈化学,与亲核试剂和亲电试剂的反应以及环加成反应。叠氮化物的1,3-偶极环加成反应是一类高度可靠且用途广泛的反应,这是由点击化学和生物正交化学的发展和迅速采用所证明的。典型的点击反应是金属催化的叠氮化物-炔烃环加成反应(Cu / RuAAC),在制药,生物医学和材料科学领域得到了广泛的应用。应变促进或畸变加速的叠氮化物-炔烃环加成消除了对金属催化剂的需要。在叠氮化物介导的1,3-偶极环加成反应中,叠氮化物是两亲性的,即HOMO-LUMO控制的偶极子, HOMO和LUMO与亲极性菌强烈相互作用。叠氮化物-炔烃的环加成反应主要是通过HOMOazide-LUMOdipolarophile相互作用进行的,缺电子的dipolarophiles反应更容易。涉及LUMOazide-HOMOdipolarophile相互作用的电子逆反应很少见,因为缺乏电子的叠氮化物(如酰基,磺酰基和磷酰基叠氮化物)的稳定性低。然而,自1960年代以来已有报道显示贫电子叠氮化物和富电子偶极亲电子之间的反应动力学增强。我们的实验室已经开发出将全氟芳基叠氮化物(PFAA)(一类稳定的缺电子叠氮化物)用作腈的前体,并用于与亲核试剂和富电子的双极性亲核试剂反应。芳基环上的全氟化还促进了PFAA的合成和产物的19F NMR光谱定量分析。在这个帐户中,我们总结了涉及亲电子叠氮化物的关键反应以及这些反应在材料合成和化学生物学中的应用。这些缺乏电子的叠氮化物对亲核试剂和富电子或应变的偶极亲电子具有独特的反应性,在某些情况下会导致新的转化,不需要任何非亲电子叠氮化物无法获得的催化剂或产物。我们重点介绍了实验室在PFAA与烯胺,烯醇化物,硫代酸和膦反应方面的工作。在PFAA与烯胺或烯醇化物的反应中,三唑或三唑啉环加成产物经过进一步的重排,以最终产物am或酰胺的速度比其非氟化对讲物快105倍。通过变形/相互作用活化应变模型进行的计算研究表明,全氟化通过降低反应物达到过渡态的变形能而降低了芳基叠氮化物的LUMO以及反应的总活化能。PFAA-烯胺反应可以使用容易获得的醛和胺原料以一锅法进行,从而使该反应特别有吸引力,例如,在纳米材料的功能化和用于制备治疗性纳米药物的抗生素的衍生化方面。对于PPAA介导的Staudinger反应,也观察到了相似的快速动力学,其反应速率比经典Staudinger连接高104倍,从而以高收率得到稳定的磷酸亚胺。该反应是双正交的,允许细胞表面标记具有最小的背景噪音。
更新日期:2020-04-23
中文翻译:

用于材料合成和化学生物学的亲电子叠氮化物。
概论有机叠氮化物参与多种有用的转化,包括腈化学,与亲核试剂和亲电试剂的反应以及环加成反应。叠氮化物的1,3-偶极环加成反应是一类高度可靠且用途广泛的反应,这是由点击化学和生物正交化学的发展和迅速采用所证明的。典型的点击反应是金属催化的叠氮化物-炔烃环加成反应(Cu / RuAAC),在制药,生物医学和材料科学领域得到了广泛的应用。应变促进或畸变加速的叠氮化物-炔烃环加成消除了对金属催化剂的需要。在叠氮化物介导的1,3-偶极环加成反应中,叠氮化物是两亲性的,即HOMO-LUMO控制的偶极子, HOMO和LUMO与亲极性菌强烈相互作用。叠氮化物-炔烃的环加成反应主要是通过HOMOazide-LUMOdipolarophile相互作用进行的,缺电子的dipolarophiles反应更容易。涉及LUMOazide-HOMOdipolarophile相互作用的电子逆反应很少见,因为缺乏电子的叠氮化物(如酰基,磺酰基和磷酰基叠氮化物)的稳定性低。然而,自1960年代以来已有报道显示贫电子叠氮化物和富电子偶极亲电子之间的反应动力学增强。我们的实验室已经开发出将全氟芳基叠氮化物(PFAA)(一类稳定的缺电子叠氮化物)用作腈的前体,并用于与亲核试剂和富电子的双极性亲核试剂反应。芳基环上的全氟化还促进了PFAA的合成和产物的19F NMR光谱定量分析。在这个帐户中,我们总结了涉及亲电子叠氮化物的关键反应以及这些反应在材料合成和化学生物学中的应用。这些缺乏电子的叠氮化物对亲核试剂和富电子或应变的偶极亲电子具有独特的反应性,在某些情况下会导致新的转化,不需要任何非亲电子叠氮化物无法获得的催化剂或产物。我们重点介绍了实验室在PFAA与烯胺,烯醇化物,硫代酸和膦反应方面的工作。在PFAA与烯胺或烯醇化物的反应中,三唑或三唑啉环加成产物经过进一步的重排,以最终产物am或酰胺的速度比其非氟化对讲物快105倍。通过变形/相互作用活化应变模型进行的计算研究表明,全氟化通过降低反应物达到过渡态的变形能而降低了芳基叠氮化物的LUMO以及反应的总活化能。PFAA-烯胺反应可以使用容易获得的醛和胺原料以一锅法进行,从而使该反应特别有吸引力,例如,在纳米材料的功能化和用于制备治疗性纳米药物的抗生素的衍生化方面。对于PPAA介导的Staudinger反应,也观察到了相似的快速动力学,其反应速率比经典Staudinger连接高104倍,从而以高收率得到稳定的磷酸亚胺。该反应是双正交的,允许细胞表面标记具有最小的背景噪音。











































 京公网安备 11010802027423号
京公网安备 11010802027423号