当前位置:
X-MOL 学术
›
ACS Catal.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Elucidating the Oxygen Activation Mechanism on Ceria-Supported Copper-Oxo Species Using Time-Resolved X-ray Absorption Spectroscopy
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-03-25 , DOI: 10.1021/acscatal.0c00551 Olga V. Safonova 1 , Alexander Guda 2 , Yury Rusalev 2 , René Kopelent 1 , Grigory Smolentsev 1 , Wey Yang Teoh 3 , Jeroen A. van Bokhoven 1, 4 , Maarten Nachtegaal 1
ACS Catalysis ( IF 11.3 ) Pub Date : 2020-03-25 , DOI: 10.1021/acscatal.0c00551 Olga V. Safonova 1 , Alexander Guda 2 , Yury Rusalev 2 , René Kopelent 1 , Grigory Smolentsev 1 , Wey Yang Teoh 3 , Jeroen A. van Bokhoven 1, 4 , Maarten Nachtegaal 1
Affiliation
|
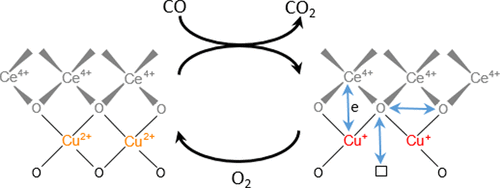
Copper-ceria finds applications in various energy-related and environmental catalysts. However, the versatile structure and complex redox activity of this material entangle uncovering structure–activity relationships and distinguishing active species from spectators. In this work, we monitored the dynamic structure of the active sites in a catalyst containing highly dispersed copper-oxo species on ceria during low-temperature CO oxidation using time-resolved X-ray absorption spectroscopy. We quantitatively demonstrate that the CO oxidation mechanism below 90 °C involves an oxygen intermediate strongly bound to the active sites as well as the redox activity of Cu2+/Cu+ and Ce4+/Ce3+ couples. The redox activity of cerium is much lower than that of copper; however, both metals change their oxidation states in concert, indicating that oxygen activation involves copper–oxo species in close interaction with ceria. In addition to short-lived Cu+ and Ce3+ intermediates that are generated in the CO oxidation cycle, long-lived Cu+ and Ce3+ species appear in the catalyst under the working conditions. We demonstrate that they do not participate in the main low-temperature CO oxidation mechanism, which is mediated by a strongly bound oxygen intermediate. Finally, our results confirm the high potential of element-specific time-resolved X-ray spectroscopy methods combined with a non-steady-state experimental strategy to uncover the mechanisms of catalytic processes in complex multicomponent systems.
中文翻译:

使用时间分辨X射线吸收光谱阐明二氧化铈负载的铜氧物种的氧活化机理
氧化铈在各种能源相关和环境催化剂中都有应用。但是,这种材料的通用结构和复杂的氧化还原活性纠缠在一起,揭示了结构与活性之间的关系,并将活性物种与观众区分开。在这项工作中,我们使用时间分辨X射线吸收光谱法监测了低温CO氧化过程中二氧化铈上含有高度分散的铜-氧物种的催化剂中活性位点的动态结构。我们定量证明,低于90°C的CO氧化机理涉及牢固结合到活性位点的氧中间体以及Cu 2+ / Cu +和Ce 4+ / Ce 3+的氧化还原活性。夫妻。铈的氧化还原活性远低于铜。然而,这两种金属的氧化态一致改变,表明氧活化涉及与氧化铈紧密相互作用的铜-氧物种。除了在CO氧化循环中生成的短寿命Cu +和Ce 3+中间体外,长寿命Cu +和Ce 3+在工作条件下,这些物种会出现在催化剂中。我们证明,他们不参与主要的低温CO氧化机制,这是由牢固结合的氧中间体介导的。最后,我们的结果证实了特定于元素的时间分辨X射线光谱法与非稳态实验策略相结合的潜力,以揭示复杂多组分系统中催化过程的机理。
更新日期:2020-04-23
中文翻译:

使用时间分辨X射线吸收光谱阐明二氧化铈负载的铜氧物种的氧活化机理
氧化铈在各种能源相关和环境催化剂中都有应用。但是,这种材料的通用结构和复杂的氧化还原活性纠缠在一起,揭示了结构与活性之间的关系,并将活性物种与观众区分开。在这项工作中,我们使用时间分辨X射线吸收光谱法监测了低温CO氧化过程中二氧化铈上含有高度分散的铜-氧物种的催化剂中活性位点的动态结构。我们定量证明,低于90°C的CO氧化机理涉及牢固结合到活性位点的氧中间体以及Cu 2+ / Cu +和Ce 4+ / Ce 3+的氧化还原活性。夫妻。铈的氧化还原活性远低于铜。然而,这两种金属的氧化态一致改变,表明氧活化涉及与氧化铈紧密相互作用的铜-氧物种。除了在CO氧化循环中生成的短寿命Cu +和Ce 3+中间体外,长寿命Cu +和Ce 3+在工作条件下,这些物种会出现在催化剂中。我们证明,他们不参与主要的低温CO氧化机制,这是由牢固结合的氧中间体介导的。最后,我们的结果证实了特定于元素的时间分辨X射线光谱法与非稳态实验策略相结合的潜力,以揭示复杂多组分系统中催化过程的机理。











































 京公网安备 11010802027423号
京公网安备 11010802027423号