当前位置:
X-MOL 学术
›
ACS Chem. Biol.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Characterization of Tailoring Steps of Nargenicin A1 Biosynthesis Reveals a Novel Analogue with Anticancer Activities.
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-03-25 , DOI: 10.1021/acschembio.9b01034 Dipesh Dhakal 1 , Jang Mi Han 1 , Ravindra Mishra 1 , Ramesh Prasad Pandey 1 , Tae-Su Kim 1 , Vijay Rayamajhi 1 , Hye Jin Jung 1, 2 , Tokutaro Yamaguchi 1, 2 , Jae Kyung Sohng 1, 2
ACS Chemical Biology ( IF 3.5 ) Pub Date : 2020-03-25 , DOI: 10.1021/acschembio.9b01034 Dipesh Dhakal 1 , Jang Mi Han 1 , Ravindra Mishra 1 , Ramesh Prasad Pandey 1 , Tae-Su Kim 1 , Vijay Rayamajhi 1 , Hye Jin Jung 1, 2 , Tokutaro Yamaguchi 1, 2 , Jae Kyung Sohng 1, 2
Affiliation
|
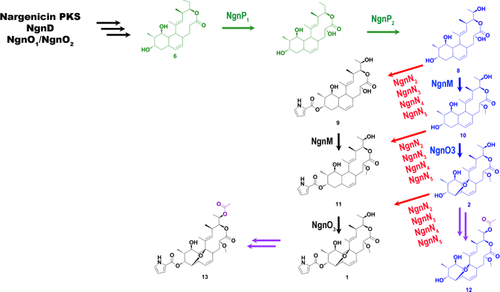
Nargenicin A1(1) is an antibacterial macrolide with effective activity against various Gram-positive bacteria, including methicillin-resistant Staphylococcus aureus. Due to the promising properties of this compound in inhibiting cell proliferation, immunomodulation, and the cell protective effect, there has been significant interest in this molecule. Recently, the biosynthetic gene cluster (BGC) of 1 was reported from Nocardia argentinesis and Nocardia arthritidis. In addition, two crucial enzymes involved in the formation of the core decalin moiety and postmodification of the decalin moiety by an ether bridge were characterized. This study reports on the BGC of 1 from Nocardia sp. CS682. In addition, the direct capture and heterologous expression of nar BGC from Nocardia sp. CS682 in Streptomyces venezuelae led to the production of 1. Further metabolic profiling of wild type, Nocardia sp. CS682 in optimized media (DD media) resulted in the isolation of two acetylated derivatives, 18-O-acetyl-nodusmicin and 18-O-acetyl-nargenicin. The post-PKS modification pathway in biosynthesis of 1 was also deciphered by identifying intermediates and/or in vitro enzymatic reactions of NgnP1, NgnM, and NgnO3. Different novel analogues of 1, such as compound 6, compound 7, 23-demethyl 8,13-deoxy-nodusmicin (8), 23-demethyl 8,13-deoxynargenicin (9), 8,13-deoxynodusmicin (10), and 8,13-deoxynargenicin (11), were also characterized, which extended our understanding of key post-PKS modification steps during the biosynthesis of 1. In addition, the antimicrobial and anticancer activities of selected analogues were also evaluated, whereas compound 9 was shown to exhibit potent antitumor activity by induction of G2/M cell cycle arrest, apoptosis, and autophagy.
中文翻译:

柚木素A1生物合成的定制步骤的表征揭示了一种具有抗癌活性的新型类似物。
Nargenicin A1(1)是一种抗菌大环内酯类,对多种革兰氏阳性细菌(包括耐甲氧西林的金黄色葡萄球菌)具有有效的活性。由于该化合物在抑制细胞增殖,免疫调节和细胞保护作用方面有希望的特性,因此对该分子引起了极大的兴趣。最近,从阿根廷诺卡氏菌和关节炎诺卡氏菌报道了1的生物合成基因簇(BGC)。另外,表征了两种核心酶,它们参与核心十氢萘部分的形成和十氢萘部分通过醚桥的后修饰。这项研究的报告BGC 1从诺卡氏菌 CS682。此外,从诺卡氏菌的nar BGC的直接捕获和异源表达。委内瑞拉链霉菌CS682的产生1。野生型诺卡氏菌的进一步代谢分析。在优化培养基(DD培养基)中的CS682导致分离出两种乙酰化衍生物18 - O-乙酰基-诺地米星和18 - O-乙酰基-纳金霉素。通过鉴定NgnP1,NgnM和NgnO3的中间体和/或体外酶促反应,还可以解释1的生物合成中的PKS后修饰途径。1的不同新颖类似物如化合物6,化合物7,23脱甲基-8,13- deoxynodusmicin(8),23脱甲基-8,13- deoxynargenicin(9),8,13-deoxynodusmicin(10),和-8,13- deoxynargenicin (11),还被表征,这扩展了我们对关键的后PKS修饰步骤的生物合成1的理解。此外,还评估了所选类似物的抗微生物和抗癌活性,而化合物9通过诱导G2 / M细胞周期停滞,凋亡和自噬而显示出强大的抗肿瘤活性。
更新日期:2020-03-25
中文翻译:

柚木素A1生物合成的定制步骤的表征揭示了一种具有抗癌活性的新型类似物。
Nargenicin A1(1)是一种抗菌大环内酯类,对多种革兰氏阳性细菌(包括耐甲氧西林的金黄色葡萄球菌)具有有效的活性。由于该化合物在抑制细胞增殖,免疫调节和细胞保护作用方面有希望的特性,因此对该分子引起了极大的兴趣。最近,从阿根廷诺卡氏菌和关节炎诺卡氏菌报道了1的生物合成基因簇(BGC)。另外,表征了两种核心酶,它们参与核心十氢萘部分的形成和十氢萘部分通过醚桥的后修饰。这项研究的报告BGC 1从诺卡氏菌 CS682。此外,从诺卡氏菌的nar BGC的直接捕获和异源表达。委内瑞拉链霉菌CS682的产生1。野生型诺卡氏菌的进一步代谢分析。在优化培养基(DD培养基)中的CS682导致分离出两种乙酰化衍生物18 - O-乙酰基-诺地米星和18 - O-乙酰基-纳金霉素。通过鉴定NgnP1,NgnM和NgnO3的中间体和/或体外酶促反应,还可以解释1的生物合成中的PKS后修饰途径。1的不同新颖类似物如化合物6,化合物7,23脱甲基-8,13- deoxynodusmicin(8),23脱甲基-8,13- deoxynargenicin(9),8,13-deoxynodusmicin(10),和-8,13- deoxynargenicin (11),还被表征,这扩展了我们对关键的后PKS修饰步骤的生物合成1的理解。此外,还评估了所选类似物的抗微生物和抗癌活性,而化合物9通过诱导G2 / M细胞周期停滞,凋亡和自噬而显示出强大的抗肿瘤活性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号