当前位置:
X-MOL 学术
›
Cryst. Growth Des.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Solvent Influence in Obtaining Diverse Coordination Symmetries of Dy(III) Metal Centers in Coordination Polymers: Synthesis, Characterization, and Luminescent Properties
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2020-03-25 , DOI: 10.1021/acs.cgd.9b01599 Nithi Phukan 1 , Soumyabrata Goswami 2 , Sophie Lipstman 1 , Israel Goldberg 1 , Bharat Kumar Tripuramallu 3
Crystal Growth & Design ( IF 3.2 ) Pub Date : 2020-03-25 , DOI: 10.1021/acs.cgd.9b01599 Nithi Phukan 1 , Soumyabrata Goswami 2 , Sophie Lipstman 1 , Israel Goldberg 1 , Bharat Kumar Tripuramallu 3
Affiliation
|
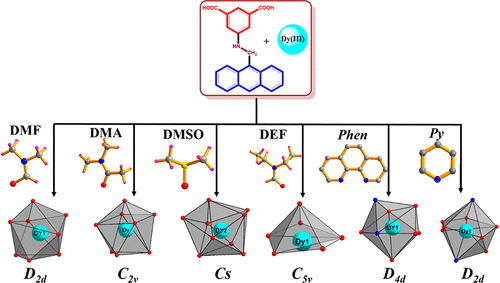
Six new Dy(III)-containing coordination polymers (CPs) have been synthesized emphasizing the role of solvent molecules in directing the formation of the Dy(III) coordination sphere which in turn influences the dimensionality of the coordination polymer, thermal stability, and emission properties. An isophthalate-based flexible ligand 5-[(anthracen-9-ylmethyl)-amino]-isophthalic acid (H2L) with different solvents such as dimethylformamide (DMF), dimethylacetamide (DMA), dimethyl sulfoxide (DMSO), diethylformamide (DEF), and N-donor linkers phen (1,10-phenanothroline) and pyridine (py) was employed to obtain six different coordination polymers, viz., [Dy2(L)2(HCOO)3(DMF)·Me2NH2·H2O]n (1), [Dy4(L)6(DMA)2(H2O)4·4DMA·4H2O]n (2), [Dy2(L)3(DMSO)3(H2O)2·2DMSO·2H2O]n (3), [{Dy(L)(DEF)}2(μ2-OH)]n (4), [Dy2(L)3(phen)2·DMA·7H2O]n (5), and [Dy(L)(NO3)(py)2·py]n (6), respectively. All the compounds were characterized by IR spectroscopy and thermogravimetric analysis, and unambiguously characterized by single crystal X-ray crystallography. The bulky anthracenyl group of the linker H2L in combination with the solvent molecules significantly alters the coordination symmetry of the Dy(III) coordination sphere in the polymers in the manner: D2d in 1, C2v and D4d in 2, C4v and Cs in 3, C5v in 4, D4d in 5, and D2d in 6. The coordination symmetry plays an interesting role in sensitization of lanthanide emission. The emission maxima of compounds 1–6 vary from the blue region to the yellow region significantly due to coordination symmetry expressed by the Dy(III) centers, which in turn depends upon the coordinated solvent molecule. The thermogravimetric profile indicates the thermal stability of the solvent free compounds up to 300 °C.
中文翻译:

获得配位聚合物中Dy(III)金属中心的各种配位对称性的溶剂影响:合成,表征和发光性能。
已经合成了六种新的含Dy(III)的配位聚合物(CP),其中强调了溶剂分子在指导Dy(III)配位球的形成中的作用,而Dy(III)配位球又会影响配位聚合物的尺寸,热稳定性和发射属性。一种基于间苯二甲酸酯的柔性配体5-[(蒽-9-基甲基)-氨基]-间苯二甲酸(H 2 L),具有不同的溶剂,例如二甲基甲酰胺(DMF),二甲基乙酰胺(DMA),二甲基亚砜(DMSO),二乙基甲酰胺( DEF)和N供体接头phen(1,10-phenanothroline)和吡啶(py)用于获得六种不同的配位聚合物,即[Dy 2(L)2(HCOO)3(DMF)·Me 2 NH2 ·H 2 O] n(1),[Dy 4(L)6(DMA)2(H 2 O)4 ·4DMA·4H 2 O] n(2),[Dy 2(L)3(DMSO)3(H 2 O)2 ·2DMSO·2H 2 O] ñ(3),[{镝(L)(DEF)} 2(μ 2 -OH)] ñ(4),[镝2(L)3( phen)2 ·DMA·7H 2O] n(5)和[Dy(L)(NO 3)(py)2 ·py] n(6)。所有化合物均通过红外光谱和热重分析进行表征,并通过单晶X射线晶体学进行明确表征。接头H 2 L的庞大蒽基与溶剂分子的结合以下列方式显着改变了聚合物中Dy(III)配位球的配位对称性:D 2 d in 1,C 2 v和D 4 d in 2,C 4 v和C s in 3,C 5 v in 4,D 4 d in 5和D 2 d in 6。配位对称在镧系元素发射的敏化中起着有趣的作用。化合物1 – 6的最大排放量由于由Dy(III)中心表示的配位对称性,蓝色区域到黄色区域的变化显着,这取决于配位的溶剂分子。热重曲线表明最高300°C的无溶剂化合物的热稳定性。
更新日期:2020-03-25
中文翻译:

获得配位聚合物中Dy(III)金属中心的各种配位对称性的溶剂影响:合成,表征和发光性能。
已经合成了六种新的含Dy(III)的配位聚合物(CP),其中强调了溶剂分子在指导Dy(III)配位球的形成中的作用,而Dy(III)配位球又会影响配位聚合物的尺寸,热稳定性和发射属性。一种基于间苯二甲酸酯的柔性配体5-[(蒽-9-基甲基)-氨基]-间苯二甲酸(H 2 L),具有不同的溶剂,例如二甲基甲酰胺(DMF),二甲基乙酰胺(DMA),二甲基亚砜(DMSO),二乙基甲酰胺( DEF)和N供体接头phen(1,10-phenanothroline)和吡啶(py)用于获得六种不同的配位聚合物,即[Dy 2(L)2(HCOO)3(DMF)·Me 2 NH2 ·H 2 O] n(1),[Dy 4(L)6(DMA)2(H 2 O)4 ·4DMA·4H 2 O] n(2),[Dy 2(L)3(DMSO)3(H 2 O)2 ·2DMSO·2H 2 O] ñ(3),[{镝(L)(DEF)} 2(μ 2 -OH)] ñ(4),[镝2(L)3( phen)2 ·DMA·7H 2O] n(5)和[Dy(L)(NO 3)(py)2 ·py] n(6)。所有化合物均通过红外光谱和热重分析进行表征,并通过单晶X射线晶体学进行明确表征。接头H 2 L的庞大蒽基与溶剂分子的结合以下列方式显着改变了聚合物中Dy(III)配位球的配位对称性:D 2 d in 1,C 2 v和D 4 d in 2,C 4 v和C s in 3,C 5 v in 4,D 4 d in 5和D 2 d in 6。配位对称在镧系元素发射的敏化中起着有趣的作用。化合物1 – 6的最大排放量由于由Dy(III)中心表示的配位对称性,蓝色区域到黄色区域的变化显着,这取决于配位的溶剂分子。热重曲线表明最高300°C的无溶剂化合物的热稳定性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号