当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Kinetic Control in the Synthesis of a Möbius Tris((ethynyl)[5]helicene) Macrocycle Using Alkyne Metathesis
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-03-25 , DOI: 10.1021/jacs.0c01430 Xing Jiang 1 , Summer D. Laffoon 2 , Dandan Chen 3 , Salvador Pérez-Estrada 4 , Andrew S. Danis 2, 5 , Joaquín Rodríguez-López 1, 2, 5 , Miguel A. Garcia-Garibay 4 , Jun Zhu 3 , Jeffrey S. Moore 1, 2, 5
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-03-25 , DOI: 10.1021/jacs.0c01430 Xing Jiang 1 , Summer D. Laffoon 2 , Dandan Chen 3 , Salvador Pérez-Estrada 4 , Andrew S. Danis 2, 5 , Joaquín Rodríguez-López 1, 2, 5 , Miguel A. Garcia-Garibay 4 , Jun Zhu 3 , Jeffrey S. Moore 1, 2, 5
Affiliation
|
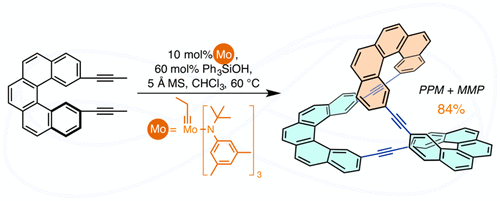
The synthesis of conjugated Möbius molecules remains elusive since twisted and macrocyclic structures are low entropy species sporting their own synthetic challenges. Here we report the synthesis of a Möbius macrocycle in 84% yield from the alkyne metathesis of 2,13-bispropynyl[5]helicene. MALDI-MS, NMR, and X-ray diffraction indicated a trimeric product of two-fold symmetry with PPM/MMP configurations in the helicene subunits. Alternatively, a three-fold symmetric, PPP/MMM structure was determined by DFT calculation to be more thermodynamically stable, illustrating remarkable kinetic selectivity for this alkyne metathesis cyclooligomerization. Computational studies provided insight into the kinetic selectivity, demonstrating a difference of 15.4 kcal/mol in activation barriers between the PPM/MMP vs. PPP/MMM diastereodetermining steps. Computational (ACID and EDDB) and experimental (UV-Vis and fluorescence spectroscopy and cyclic voltammetry) studies revealed weak conjugation between the alkyne and adjacent helicene groups, as well as the lack of significant global aromaticity. The separation of PPM/MMP enantiomers was achieved via chiral HPLC at the analytical scale.
中文翻译:

使用炔复分解合成莫比乌斯三((乙炔基)[5] 螺旋烯)大环的动力学控制
共轭莫比乌斯分子的合成仍然难以捉摸,因为扭曲和大环结构是低熵物种,面临着自己的合成挑战。在这里,我们报告了从 2,13-双丙炔基 [5] 螺旋的炔复分解反应以 84% 的产率合成莫比乌斯大环。MALDI-MS、NMR 和 X 射线衍射表明螺旋烯亚基中具有 PPM/MMP 构型的双重对称性三聚体产物。或者,通过 DFT 计算确定了三重对称的 PPP/MMM 结构在热力学上更加稳定,说明这种炔烃复分解环低聚具有显着的动力学选择性。计算研究提供了对动力学选择性的深入了解,证明 PPM/MMP 与 PPP/MMM 非对映测定步骤之间的活化势垒差异为 15.4 kcal/mol。计算(ACID 和 EDDB)和实验(UV-Vis 和荧光光谱和循环伏安法)研究表明炔烃和相邻螺旋基团之间的共轭较弱,并且缺乏显着的全局芳香性。PPM/MMP 对映异构体的分离是通过分析规模的手性 HPLC 实现的。
更新日期:2020-03-25
中文翻译:

使用炔复分解合成莫比乌斯三((乙炔基)[5] 螺旋烯)大环的动力学控制
共轭莫比乌斯分子的合成仍然难以捉摸,因为扭曲和大环结构是低熵物种,面临着自己的合成挑战。在这里,我们报告了从 2,13-双丙炔基 [5] 螺旋的炔复分解反应以 84% 的产率合成莫比乌斯大环。MALDI-MS、NMR 和 X 射线衍射表明螺旋烯亚基中具有 PPM/MMP 构型的双重对称性三聚体产物。或者,通过 DFT 计算确定了三重对称的 PPP/MMM 结构在热力学上更加稳定,说明这种炔烃复分解环低聚具有显着的动力学选择性。计算研究提供了对动力学选择性的深入了解,证明 PPM/MMP 与 PPP/MMM 非对映测定步骤之间的活化势垒差异为 15.4 kcal/mol。计算(ACID 和 EDDB)和实验(UV-Vis 和荧光光谱和循环伏安法)研究表明炔烃和相邻螺旋基团之间的共轭较弱,并且缺乏显着的全局芳香性。PPM/MMP 对映异构体的分离是通过分析规模的手性 HPLC 实现的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号