当前位置:
X-MOL 学术
›
J. Am. Chem. Soc.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Unraveling Reaction Mechanisms of Mo2C as Cathode Catalyst in Li-CO2 Battery
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-03-25 , DOI: 10.1021/jacs.9b12868 Chao Yang 1 , Kunkun Guo 1 , Dingwang Yuan 1 , Jianli Cheng 2 , Bin Wang 2
Journal of the American Chemical Society ( IF 14.4 ) Pub Date : 2020-03-25 , DOI: 10.1021/jacs.9b12868 Chao Yang 1 , Kunkun Guo 1 , Dingwang Yuan 1 , Jianli Cheng 2 , Bin Wang 2
Affiliation
|
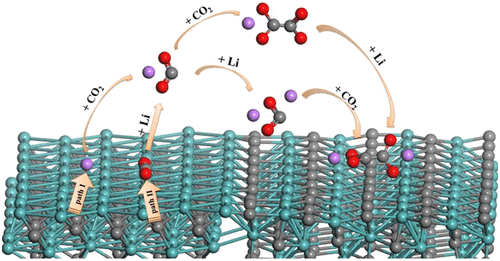
First-principles density functional theory (DFT) calculations are firstly used to study possible reaction mechanisms of molybdenum carbide (Mo2C) as cathode catalysts in Li-CO2 batteries. By systematically investigating the Gibbs free energy changes of different intermediates during lithium oxalate (Li2C2O4) and lithium carbonate (Li2CO3) nucleations, it is theoretically demonstrated that Li2C2O4 could be stabilized as the final discharge product, preventing the further formation of Li2CO3. The surface charge distributions of Li2C2O4 adsorbing onto catalytic surfaces are studied by using Bader charge analysis, giving that electron transfers are found between Li2C2O4 and Mo2C surfaces. The catalytic activities of catalysts are intensively evaluated toward the discharge and charge processes by calculating the electrochemical free energy diagrams to identify the overpotentials. Our studies would promote the understanding of electrochemical processes and shed more light on the design and optimization cathode catalysts for Li-CO2 batteries.
中文翻译:

揭示 Mo2C 作为 Li-CO2 电池阴极催化剂的反应机理
第一性原理密度泛函理论(DFT)计算首先用于研究碳化钼(Mo2C)作为锂-二氧化碳电池阴极催化剂的可能反应机制。通过系统地研究草酸锂 (Li2C2O4) 和碳酸锂 (Li2CO3) 成核过程中不同中间体的吉布斯自由能变化,理论上证明了 Li2C2O4 可以稳定作为最终放电产物,防止进一步形成 Li2CO3。通过使用巴德电荷分析研究吸附在催化表面上的 Li2C2O4 的表面电荷分布,发现在 Li2C2O4 和 Mo2C 表面之间存在电子转移。通过计算电化学自由能图来识别过电位,对放电和充电过程中催化剂的催化活性进行了深入评估。我们的研究将促进对电化学过程的理解,并为 Li-CO2 电池的阴极催化剂的设计和优化提供更多信息。
更新日期:2020-03-25
中文翻译:

揭示 Mo2C 作为 Li-CO2 电池阴极催化剂的反应机理
第一性原理密度泛函理论(DFT)计算首先用于研究碳化钼(Mo2C)作为锂-二氧化碳电池阴极催化剂的可能反应机制。通过系统地研究草酸锂 (Li2C2O4) 和碳酸锂 (Li2CO3) 成核过程中不同中间体的吉布斯自由能变化,理论上证明了 Li2C2O4 可以稳定作为最终放电产物,防止进一步形成 Li2CO3。通过使用巴德电荷分析研究吸附在催化表面上的 Li2C2O4 的表面电荷分布,发现在 Li2C2O4 和 Mo2C 表面之间存在电子转移。通过计算电化学自由能图来识别过电位,对放电和充电过程中催化剂的催化活性进行了深入评估。我们的研究将促进对电化学过程的理解,并为 Li-CO2 电池的阴极催化剂的设计和优化提供更多信息。











































 京公网安备 11010802027423号
京公网安备 11010802027423号