当前位置:
X-MOL 学术
›
ChemPhysChem
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
On the Stability of Interactions between Pairs of Anions - Complexes of MCl3 - (M=Be, Mg, Ca, Sr, Ba) with Pyridine and CN.
ChemPhysChem ( IF 2.3 ) Pub Date : 2020-03-23 , DOI: 10.1002/cphc.202000098 Wiktor Zierkiewicz 1 , Rafał Wysokiński 1 , Mariusz Michalczyk 1 , Steve Scheiner 2
ChemPhysChem ( IF 2.3 ) Pub Date : 2020-03-23 , DOI: 10.1002/cphc.202000098 Wiktor Zierkiewicz 1 , Rafał Wysokiński 1 , Mariusz Michalczyk 1 , Steve Scheiner 2
Affiliation
|
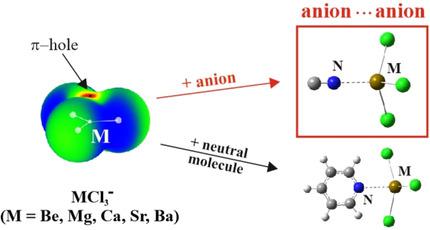
The ability of the central M atom of the MCl3− anion, with M=Be, Mg, Ca, Sr, Ba, to engage in a noncovalent bond with an approaching nucleophile is gauged by ab initio methods. The N atom of pyridine forms a M⋅⋅⋅N bond with an interaction energy between 12 and 21 kcal mol−1, even though the π‐hole above the M atom is not necessarily positive in sign. Despite a strong Coulombic repulsion between two anions, CN− is also able to approach the M atom so as to engage in a metastable complex that is higher in energy than the individual anions. The energy barrier separating this complex from its constituent anion pair is roughly 20 kcal mol−1. Despite the endothermic formation reaction energy of the CN−⋅⋅⋅MCl3− complex, the electron topology signals a strong interaction, more so than in pyridine⋅⋅⋅MCl3− with its exothermic binding energy. The dianionic complex is held together largely on the strength of interorbital interactions, thereby overcoming a repulsive electrostatic component. The latter is partially alleviated by the pyramidalization of the MCl3 unit which makes its π‐hole more positive. The complex sinks below the separate monomers in energy when the system is immersed in an aqueous medium, with a binding energy that varies from as much as 20 kcal mol−1 for Be down to 1.2 kcal mol−1 for Ba.
中文翻译:

MCl3-(M = Be,Mg,Ca,Sr,Ba)与吡啶和CN的阴离子对-配合物之间相互作用的稳定性。
所述的MC1的,中心M原子的能力3 -阴离子参与与一个亲核试剂接近非共价键,其中M =铍,镁,钙,锶,钡,由从头方法衡量。吡啶的N原子形成M⋅⋅⋅N键,其相互作用能在12至21 kcal mol -1之间,即使M原子上方的π孔不一定是正号。在战胜两场阴离子,CN之间有很强的库仑斥力-也能够接近M个原子,从而在亚复杂的是在能量比个人更高的阴离子参与。将该络合物与其构成的阴离子对分离的能垒约为20 kcal mol -1。尽管CN的吸热形成反应的能量-⋅⋅⋅MCl 3 -复杂,电子拓扑信号的强的相互作用,更使比pyridine⋅⋅⋅MCl 3 -其放热的结合能。双阴离子络合物在轨道间相互作用的强度上很大程度上保持在一起,从而克服了排斥性静电成分。后者由于MCl 3单元的锥体化而部分缓解,这使其π孔更正。当系统浸入水性介质中时,络合物的能量陷入单独的单体之下,其结合能从Be的高达20 kcal mol -1降至Ba的1.2 kcal mol -1。
更新日期:2020-03-23
中文翻译:

MCl3-(M = Be,Mg,Ca,Sr,Ba)与吡啶和CN的阴离子对-配合物之间相互作用的稳定性。
所述的MC1的,中心M原子的能力3 -阴离子参与与一个亲核试剂接近非共价键,其中M =铍,镁,钙,锶,钡,由从头方法衡量。吡啶的N原子形成M⋅⋅⋅N键,其相互作用能在12至21 kcal mol -1之间,即使M原子上方的π孔不一定是正号。在战胜两场阴离子,CN之间有很强的库仑斥力-也能够接近M个原子,从而在亚复杂的是在能量比个人更高的阴离子参与。将该络合物与其构成的阴离子对分离的能垒约为20 kcal mol -1。尽管CN的吸热形成反应的能量-⋅⋅⋅MCl 3 -复杂,电子拓扑信号的强的相互作用,更使比pyridine⋅⋅⋅MCl 3 -其放热的结合能。双阴离子络合物在轨道间相互作用的强度上很大程度上保持在一起,从而克服了排斥性静电成分。后者由于MCl 3单元的锥体化而部分缓解,这使其π孔更正。当系统浸入水性介质中时,络合物的能量陷入单独的单体之下,其结合能从Be的高达20 kcal mol -1降至Ba的1.2 kcal mol -1。











































 京公网安备 11010802027423号
京公网安备 11010802027423号