当前位置:
X-MOL 学术
›
Eur. J. Org. Chem.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Short-chained Anthracene Strapped Porphyrins and their Endoperoxides
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2020-04-20 , DOI: 10.1002/ejoc.202000283 Susan Callaghan 1 , Keith J Flanagan 1 , John E O'Brien 1 , Mathias O Senge 1, 2
European Journal of Organic Chemistry ( IF 2.5 ) Pub Date : 2020-04-20 , DOI: 10.1002/ejoc.202000283 Susan Callaghan 1 , Keith J Flanagan 1 , John E O'Brien 1 , Mathias O Senge 1, 2
Affiliation
|
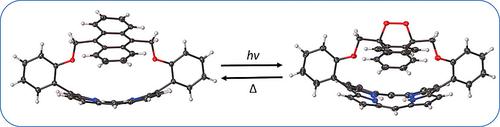
The syntheses of short‐chained anthracene‐strapped porphyrins and their Zn(II)complexes are reported. The key synthetic step is a [2+2] condensation between a dipyrromethane and an anthracene bisaldehyde, 2,2'‐((anthracene‐9,10‐diylbis(methylene))bis(oxy))dibenzaldehyde. Following exposure to white light, self‐sensitized singlet oxygen and the anthracene moieties underwent [4+2] cycloaddition reactions to yield the corresponding endoperoxides. 1H NMR studies demonstrate that the endoperoxide readily formed in [D]chloroform and decayed at 85 °C. X‐ray crystallography and absorption spectroscopy were used to confirm macrocyclic distortion in the parent strapped porphyrins and endoperoxides. Additionally, X‐ray crystallography indicated that endoperoxide formation occurred exclusively on the outside face of the anthracene moiety.
中文翻译:

短链蒽带卟啉及其内过氧化物
报道了短链蒽带卟啉及其 Zn(II) 配合物的合成。关键的合成步骤是二吡咯甲烷和蒽双醛2,2'-((蒽-9,10-二基双(亚甲基))双(氧基))二苯甲醛之间的[2+2]缩合。暴露于白光后,自敏化单线态氧和蒽部分发生[4+2]环加成反应,产生相应的内过氧化物。 1H NMR 研究表明,内过氧化物很容易在[D]氯仿中形成,并在 85 °C 下分解。 X射线晶体学和吸收光谱用于确认母体带状卟啉和内过氧化物的大环畸变。此外,X射线晶体学表明内过氧化物的形成仅发生在蒽部分的外表面上。
更新日期:2020-04-20
中文翻译:

短链蒽带卟啉及其内过氧化物
报道了短链蒽带卟啉及其 Zn(II) 配合物的合成。关键的合成步骤是二吡咯甲烷和蒽双醛2,2'-((蒽-9,10-二基双(亚甲基))双(氧基))二苯甲醛之间的[2+2]缩合。暴露于白光后,自敏化单线态氧和蒽部分发生[4+2]环加成反应,产生相应的内过氧化物。 1H NMR 研究表明,内过氧化物很容易在[D]氯仿中形成,并在 85 °C 下分解。 X射线晶体学和吸收光谱用于确认母体带状卟啉和内过氧化物的大环畸变。此外,X射线晶体学表明内过氧化物的形成仅发生在蒽部分的外表面上。











































 京公网安备 11010802027423号
京公网安备 11010802027423号