当前位置:
X-MOL 学术
›
Soft Matter
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Electrophoretic origin of long-range repulsion of colloids near water/Nafion interfaces
Soft Matter ( IF 2.9 ) Pub Date : 2020-03-23 , DOI: 10.1039/d0sm00170h Maria J. Esplandiu 1, 2, 3, 4, 5 , David Reguera 5, 6, 7, 7, 8 , Jordi Fraxedas 1, 2, 3, 4, 5
Soft Matter ( IF 2.9 ) Pub Date : 2020-03-23 , DOI: 10.1039/d0sm00170h Maria J. Esplandiu 1, 2, 3, 4, 5 , David Reguera 5, 6, 7, 7, 8 , Jordi Fraxedas 1, 2, 3, 4, 5
Affiliation
|
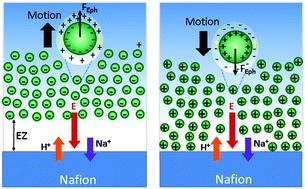
One of the most striking properties of Nafion is the formation of a long-range solute exclusion zone (EZ) in contact with water. The mechanism of formation of this EZ has been the subject of a controversial and long-standing debate. Previous studies by Schurr et al. and Florea et al. root the explanation of this phenomenon in the ion-exchange properties of Nafion, which generates ion diffusion and ion gradients that drive the repulsion of solutes by diffusiophoresis. Here we have evaluated separately the electrophoretic and chemiphoretic contributions to multi-ionic diffusiophoresis using differently charged colloidal tracers as solutes to identify better their contribution in the EZ formation. Our experimental results, which are also supported by numerical simulations, show that the electric field, built up due to the unequal diffusion coefficients of the exchanged ions, is the dominant parameter behind such interfacial phenomenon in the presence of alkali metal chlorides. The EZ formation depends on the interplay of the electric field with the zeta potential of the solute and can be additionally modulated by changing ion diffusion coefficients or adding salts. As a consequence, we show that not all solutes can be expelled from the Nafion interface and hence the EZ is not always formed. This study thus provides a more detailed description of the origin and dynamics of this phenomenon and opens the door to the rational use of this active interface for many potential applications.
中文翻译:

水/ Nafion界面附近胶体远距离排斥的电泳起源
Nafion最引人注目的特性之一是与水接触形成了一个长距离溶质排斥区(EZ)。这个EZ的形成机制一直是有争议和长期辩论的主题。Schurr等人先前的研究。和Florea等。对该现象的解释源于Nafion的离子交换特性,Nafion产生离子扩散和离子梯度,从而通过扩散电泳驱动溶质排斥。在这里,我们使用不同电荷的胶体示踪剂作为溶质,分别评估了电泳和化学电泳对多离子扩散电泳的贡献,以更好地确定它们在EZ形成中的贡献。我们的实验结果也得到了数值模拟的支持,结果表明,由于交换离子的扩散系数不相等而建立的电场是存在碱金属氯化物时发生这种界面现象的主要参数。EZ的形成取决于电场与溶质的ζ电位的相互作用,并且可以通过改变离子扩散系数或添加盐来额外调节。结果,我们表明并不是所有的溶质都能从Nafion界面排出,因此EZ并不总是形成的。因此,这项研究提供了对该现象的起源和动态的更详细描述,并为在许多潜在应用中合理使用此主动接口打开了大门。
更新日期:2020-04-24
中文翻译:

水/ Nafion界面附近胶体远距离排斥的电泳起源
Nafion最引人注目的特性之一是与水接触形成了一个长距离溶质排斥区(EZ)。这个EZ的形成机制一直是有争议和长期辩论的主题。Schurr等人先前的研究。和Florea等。对该现象的解释源于Nafion的离子交换特性,Nafion产生离子扩散和离子梯度,从而通过扩散电泳驱动溶质排斥。在这里,我们使用不同电荷的胶体示踪剂作为溶质,分别评估了电泳和化学电泳对多离子扩散电泳的贡献,以更好地确定它们在EZ形成中的贡献。我们的实验结果也得到了数值模拟的支持,结果表明,由于交换离子的扩散系数不相等而建立的电场是存在碱金属氯化物时发生这种界面现象的主要参数。EZ的形成取决于电场与溶质的ζ电位的相互作用,并且可以通过改变离子扩散系数或添加盐来额外调节。结果,我们表明并不是所有的溶质都能从Nafion界面排出,因此EZ并不总是形成的。因此,这项研究提供了对该现象的起源和动态的更详细描述,并为在许多潜在应用中合理使用此主动接口打开了大门。











































 京公网安备 11010802027423号
京公网安备 11010802027423号