当前位置:
X-MOL 学术
›
Org. Chem. Front.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Direct synthesis of annulated indoles through palladium-catalyzed double alkylations
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020-03-24 , DOI: 10.1039/d0qo00135j Yadong Gao 1, 2, 3, 4, 5 , Jianhua Li 1, 2, 3, 4 , Songlin Bai 4, 5, 6 , Daoquan Tu 1, 2, 3, 4 , Chao Yang 1, 2, 3, 4, 5 , Zhiwen Ye 1, 2, 3, 4 , Bingcheng Hu 1, 2, 3, 4 , Xiangbing Qi 4, 5, 6, 7, 8 , Chao Jiang 1, 2, 3, 4
Organic Chemistry Frontiers ( IF 4.6 ) Pub Date : 2020-03-24 , DOI: 10.1039/d0qo00135j Yadong Gao 1, 2, 3, 4, 5 , Jianhua Li 1, 2, 3, 4 , Songlin Bai 4, 5, 6 , Daoquan Tu 1, 2, 3, 4 , Chao Yang 1, 2, 3, 4, 5 , Zhiwen Ye 1, 2, 3, 4 , Bingcheng Hu 1, 2, 3, 4 , Xiangbing Qi 4, 5, 6, 7, 8 , Chao Jiang 1, 2, 3, 4
Affiliation
|
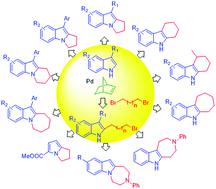
A facile, one-step synthesis of annulated indoles from (N–H) indoles and dibromoalkanes was developed through a palladium-catalyzed double alkylation process. The reaction proceeds through a palladium-catalyzed norbornene-mediated C2-alkylation of indoles and subsequent regioselective cyclization. The reaction is compatible with various indole substituents, as well as a range of simple and functionalized dibromoalkyl reagents. The detailed reaction mechanism was investigated by key intermediate experiments, NMR spectroscopic analyses, and DFT calculations. The results of a preliminary mechanistic study show a catalytic cycle consisting of initial C2-alkylation of indoles that is followed by N-alkylation or palladium promoted intramolecular C3-nucleophilic substitution to yield, respectively, [a]- or [b]-annulated indoles.
中文翻译:

通过钯催化的双烷基化直接合成环状吲哚
通过钯催化的双烷基化工艺,可以轻松地从(NH)吲哚和二溴代烷烃一步一步合成环状吲哚。该反应通过钯催化的降冰片烯介导的吲哚的C2-烷基化和随后的区域选择性环化而进行。该反应与各种吲哚取代基以及一系列简单和功能化的二溴烷基试剂兼容。通过关键的中间实验,NMR光谱分析和DFT计算研究了详细的反应机理。初步机理研究的结果表明,催化循环由最初的吲哚C2-烷基化,随后的N-烷基化或钯促进的分子内C3-亲核取代产生[[ a]-或[ b ]环吲哚。
更新日期:2020-03-24
中文翻译:

通过钯催化的双烷基化直接合成环状吲哚
通过钯催化的双烷基化工艺,可以轻松地从(NH)吲哚和二溴代烷烃一步一步合成环状吲哚。该反应通过钯催化的降冰片烯介导的吲哚的C2-烷基化和随后的区域选择性环化而进行。该反应与各种吲哚取代基以及一系列简单和功能化的二溴烷基试剂兼容。通过关键的中间实验,NMR光谱分析和DFT计算研究了详细的反应机理。初步机理研究的结果表明,催化循环由最初的吲哚C2-烷基化,随后的N-烷基化或钯促进的分子内C3-亲核取代产生[[ a]-或[ b ]环吲哚。











































 京公网安备 11010802027423号
京公网安备 11010802027423号