当前位置:
X-MOL 学术
›
Chem. Commun.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Nitrosoarene-catalyzed regioselective aromatic C-H sulfinylation with thiols under aerobic conditions.
Chemical Communications ( IF 4.3 ) Pub Date : 2020-04-05 , DOI: 10.1039/d0cc01188f Suman Pradhan 1 , Sandeep Patel , Indranil Chatterjee
Chemical Communications ( IF 4.3 ) Pub Date : 2020-04-05 , DOI: 10.1039/d0cc01188f Suman Pradhan 1 , Sandeep Patel , Indranil Chatterjee
Affiliation
|
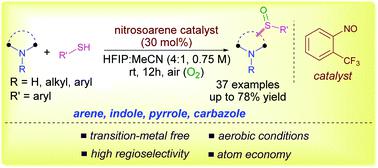
Aromatic amines and (hetero)arenes, such as indoles and pyrroles, are regioselectively sulfinylated under mild aerobic conditions using nitrosoarenes as a redox-catalyst. The nitrosoarene is involved in the electron transfer process with arenes to generate a crucial arene radical cation intermediate for C-H sulfinylation. The present methodology requires no directing group, can be scaled up easily and is applicable for the late-stage functionalization of drug molecules and natural products with high regioselectivity.
中文翻译:

在有氧条件下,亚硝基芳烃催化的区域选择性芳香族CH亚硫醇化反应。
使用亚硝基芳烃作为氧化还原催化剂,在温和的好氧条件下,将芳香胺和(杂)芳烃(如吲哚和吡咯)进行区域选择性亚磺酰化。亚硝基芳烃与芳烃一起参与电子转移过程,生成用于CH亚磺酰化的关键芳烃自由基阳离子中间体。本方法不需要指导基团,可以容易地按比例放大,并且适用于具有高区域选择性的药物分子和天然产物的后期功能化。
更新日期:2020-03-23
中文翻译:

在有氧条件下,亚硝基芳烃催化的区域选择性芳香族CH亚硫醇化反应。
使用亚硝基芳烃作为氧化还原催化剂,在温和的好氧条件下,将芳香胺和(杂)芳烃(如吲哚和吡咯)进行区域选择性亚磺酰化。亚硝基芳烃与芳烃一起参与电子转移过程,生成用于CH亚磺酰化的关键芳烃自由基阳离子中间体。本方法不需要指导基团,可以容易地按比例放大,并且适用于具有高区域选择性的药物分子和天然产物的后期功能化。











































 京公网安备 11010802027423号
京公网安备 11010802027423号