当前位置:
X-MOL 学术
›
PLOS Negl. Trop. Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Deep phosphoproteome analysis of Schistosoma mansoni leads development of a kinomic array that highlights sex-biased differences in adult worm protein phosphorylation.
PLOS Neglected Tropical Diseases ( IF 3.4 ) Pub Date : 2020-03-23 , DOI: 10.1371/journal.pntd.0008115 Natasha L Hirst 1 , Jean-Christophe Nebel 2 , Scott P Lawton 1 , Anthony J Walker 1
PLOS Neglected Tropical Diseases ( IF 3.4 ) Pub Date : 2020-03-23 , DOI: 10.1371/journal.pntd.0008115 Natasha L Hirst 1 , Jean-Christophe Nebel 2 , Scott P Lawton 1 , Anthony J Walker 1
Affiliation
|
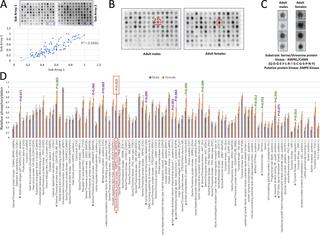
Although helminth parasites cause enormous suffering worldwide we know little of how protein phosphorylation, one of the most important post-translational modifications used for molecular signalling, regulates their homeostasis and function. This is particularly the case for schistosomes. Herein, we report a deep phosphoproteome exploration of adult Schistosoma mansoni, providing one of the richest phosphoprotein resources for any parasite so far, and employ the data to build the first parasite-specific kinomic array. Complementary phosphopeptide enrichment strategies were used to detect 15,844 unique phosphopeptides mapping to 3,176 proteins. The phosphoproteins were predicted to be involved in a wide range of biological processes and phosphoprotein interactome analysis revealed 55 highly interconnected clusters including those enriched with ribosome, proteasome, phagosome, spliceosome, glycolysis, and signalling proteins. 93 distinct phosphorylation motifs were identified, with 67 providing a 'footprint' of protein kinase activity; CaMKII, PKA and CK1/2 were highly represented supporting their central importance to schistosome function. Within the kinome, 808 phosphorylation sites were matched to 136 protein kinases, and 68 sites within 37 activation loops were discovered. Analysis of putative protein kinase-phosphoprotein interactions revealed canonical networks but also novel interactions between signalling partners. Kinomic array analysis of male and female adult worm extracts revealed high phosphorylation of transformation:transcription domain associated protein by both sexes, and CDK and AMPK peptides by females. Moreover, eight peptides including protein phosphatase 2C gamma, Akt, Rho2 GTPase, SmTK4, and the insulin receptor were more highly phosphorylated by female extracts, highlighting their possible importance to female worm function. We envision that these findings, tools and methodology will help drive new research into the functional biology of schistosomes and other helminth parasites, and support efforts to develop new therapeutics for their control.
中文翻译:

曼氏血吸虫的深入磷酸化蛋白质组学分析导致了一个动力学阵列的发展,该阵列突出了成虫蠕虫蛋白质磷酸化中的性别偏见差异。
尽管蠕虫寄生虫在世界范围内造成了巨大的痛苦,但我们对蛋白质磷酸化(用于分子信号转导的最重要的翻译后修饰之一)如何调节其稳态和功能知之甚少。血吸虫尤其如此。在这里,我们报告了对成人曼氏血吸虫的深入磷酸化蛋白质组学研究,为迄今为止的任何寄生虫提供了最丰富的磷蛋白资源之一,并利用该数据建立了第一个寄生虫特异性的动力学阵列。互补的磷酸肽富集策略用于检测15844种独特的磷酸肽,定位到3176种蛋白质上。预测磷蛋白将参与广泛的生物学过程,磷蛋白相互作用组分析显示55个高度相互连接的簇,包括富含核糖体,蛋白酶体,吞噬体,剪接体,糖酵解和信号蛋白的簇。鉴定出93个不同的磷酸化基序,其中67个提供了蛋白激酶活性的“足迹”。CaMKII,PKA和CK1 / 2被高度代表,支持其对血吸虫功能的重要作用。在激酶组中,808个磷酸化位点与136个蛋白激酶匹配,并且在37个激活环中发现了68个位点。推定的蛋白激酶-磷酸蛋白相互作用的分析揭示了规范的网络,但信号伴侣之间也有新颖的相互作用。雄性和雌性成虫蠕虫提取物的动力学阵列分析表明,性别的转化:转录域相关蛋白和雌性的CDK和AMPK肽的磷酸化水平很高。此外,雌性提取物对8种肽(包括蛋白磷酸酶2Cγ,Akt,Rho2 GTPase,SmTK4和胰岛素受体)的磷酸化程度更高,突显了它们对雌性蠕虫功能的重要性。我们认为,这些发现,工具和方法将有助于推动对血吸虫和其他蠕虫寄生虫的功能生物学的新研究,并支持为控制其发展新疗法的努力。雌性提取物对8种肽(包括蛋白磷酸酶2Cγ,Akt,Rho2 GTPase,SmTK4和胰岛素受体)的磷酸化程度更高,突显了它们对雌性蠕虫功能的重要性。我们认为,这些发现,工具和方法将有助于推动对血吸虫和其他蠕虫寄生虫的功能生物学的新研究,并支持为控制其发展新疗法的努力。雌性提取物对8种肽(包括蛋白磷酸酶2Cγ,Akt,Rho2 GTPase,SmTK4和胰岛素受体)的磷酸化程度更高,突显了它们对雌性蠕虫功能的重要性。我们认为,这些发现,工具和方法将有助于推动对血吸虫和其他蠕虫寄生虫的功能生物学的新研究,并支持为控制其发展新疗法的努力。
更新日期:2020-03-24
中文翻译:

曼氏血吸虫的深入磷酸化蛋白质组学分析导致了一个动力学阵列的发展,该阵列突出了成虫蠕虫蛋白质磷酸化中的性别偏见差异。
尽管蠕虫寄生虫在世界范围内造成了巨大的痛苦,但我们对蛋白质磷酸化(用于分子信号转导的最重要的翻译后修饰之一)如何调节其稳态和功能知之甚少。血吸虫尤其如此。在这里,我们报告了对成人曼氏血吸虫的深入磷酸化蛋白质组学研究,为迄今为止的任何寄生虫提供了最丰富的磷蛋白资源之一,并利用该数据建立了第一个寄生虫特异性的动力学阵列。互补的磷酸肽富集策略用于检测15844种独特的磷酸肽,定位到3176种蛋白质上。预测磷蛋白将参与广泛的生物学过程,磷蛋白相互作用组分析显示55个高度相互连接的簇,包括富含核糖体,蛋白酶体,吞噬体,剪接体,糖酵解和信号蛋白的簇。鉴定出93个不同的磷酸化基序,其中67个提供了蛋白激酶活性的“足迹”。CaMKII,PKA和CK1 / 2被高度代表,支持其对血吸虫功能的重要作用。在激酶组中,808个磷酸化位点与136个蛋白激酶匹配,并且在37个激活环中发现了68个位点。推定的蛋白激酶-磷酸蛋白相互作用的分析揭示了规范的网络,但信号伴侣之间也有新颖的相互作用。雄性和雌性成虫蠕虫提取物的动力学阵列分析表明,性别的转化:转录域相关蛋白和雌性的CDK和AMPK肽的磷酸化水平很高。此外,雌性提取物对8种肽(包括蛋白磷酸酶2Cγ,Akt,Rho2 GTPase,SmTK4和胰岛素受体)的磷酸化程度更高,突显了它们对雌性蠕虫功能的重要性。我们认为,这些发现,工具和方法将有助于推动对血吸虫和其他蠕虫寄生虫的功能生物学的新研究,并支持为控制其发展新疗法的努力。雌性提取物对8种肽(包括蛋白磷酸酶2Cγ,Akt,Rho2 GTPase,SmTK4和胰岛素受体)的磷酸化程度更高,突显了它们对雌性蠕虫功能的重要性。我们认为,这些发现,工具和方法将有助于推动对血吸虫和其他蠕虫寄生虫的功能生物学的新研究,并支持为控制其发展新疗法的努力。雌性提取物对8种肽(包括蛋白磷酸酶2Cγ,Akt,Rho2 GTPase,SmTK4和胰岛素受体)的磷酸化程度更高,突显了它们对雌性蠕虫功能的重要性。我们认为,这些发现,工具和方法将有助于推动对血吸虫和其他蠕虫寄生虫的功能生物学的新研究,并支持为控制其发展新疗法的努力。











































 京公网安备 11010802027423号
京公网安备 11010802027423号