当前位置:
X-MOL 学术
›
Cell Death Dis.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
IL28A protein homotetramer structure is required for autolysosomal degradation of HCV-NS5A in vitro.
Cell Death & Disease ( IF 8.1 ) Pub Date : 2020-03-23 , DOI: 10.1038/s41419-020-2400-9 Yuan-Yuan Ma 1 , Jian-Rui Li 1 , Zong-Gen Peng 1 , Jing-Pu Zhang 1
Cell Death & Disease ( IF 8.1 ) Pub Date : 2020-03-23 , DOI: 10.1038/s41419-020-2400-9 Yuan-Yuan Ma 1 , Jian-Rui Li 1 , Zong-Gen Peng 1 , Jing-Pu Zhang 1
Affiliation
|
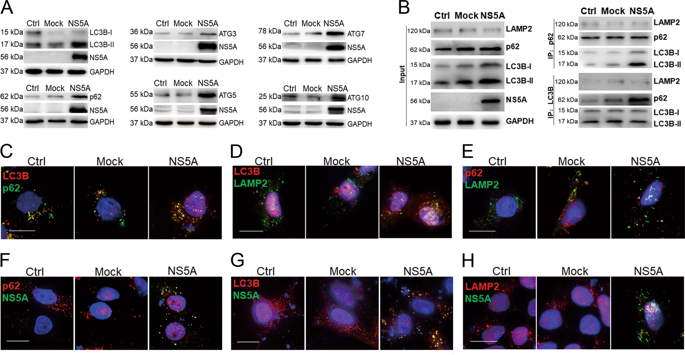
Interferon lambda-2 (IL28A) has a wide antiviral effect with fewer side-effects. Autophagy is a host mechanism to maintain intracellular homeostasis and defends invasion of pathogenic microorganisms. HCV NS5A can disable host defense systems to support HCV replication. Thus, molecular mechanism of interaction among interferon lambda, autophagy, and HCV was concerned and explored in this study. We report that HCV NS5A activated an incomplete autophagy by promoting the autophagic ubiquitylation-like enzymes ATG3, ATG5, ATG7, ATG10, and autophagosome maker LC3B, but blocked autophagy flux; IL28A bound to NS5A at NS5A-ISDR region, and degraded HCV-NS5A by promoting autolysosome formations in HepG2 cells. A software prediction of IL28A protein conformation indicated a potential structure of IL28A homotetramer; the first α-helix of IL28A locates in the interfaces among the four IL28A chains to maintain IL28A homotetrameric conformation. Co-IP and cell immunofluorescence experiments with sequential deletion mutants demonstrate that IL28A preferred a homotetramer conformation to a monomer in the cells; the IL28A homotetramer is positively correlated with autolysosomal degradation of HCV NS5A and the other HCV proteins. Summarily, the first α-helix of IL28A protein is the key domain for maintaining IL28A homotetramer which is required for promoting formation of autolysosomes and degradation of HCV proteins in vitro.
中文翻译:

IL28A 蛋白同四聚体结构是 HCV-NS5A 体外自溶酶体降解所必需的。
干扰素 lambda-2 (IL28A) 具有广泛的抗病毒作用,且副作用较少。自噬是宿主维持细胞内稳态、防御病原微生物入侵的机制。 HCV NS5A 可以禁用主机防御系统以支持 HCV 复制。因此,本研究关注并探讨干扰素λ、自噬和HCV之间相互作用的分子机制。我们报道HCV NS5A通过促进自噬泛素化样酶ATG3、ATG5、ATG7、ATG10和自噬体标记物LC3B激活不完全自噬,但阻断自噬通量; IL28A 在 NS5A-ISDR 区域与 NS5A 结合,并通过促进 HepG2 细胞中自溶酶体的形成来降解 HCV-NS5A。 IL28A 蛋白构象的软件预测表明了 IL28A 同四聚体的潜在结构; IL28A的第一个α螺旋位于四个IL28A链之间的界面以维持IL28A同源四聚体构象。使用连续缺失突变体的 Co-IP 和细胞免疫荧光实验表明,IL28A 在细胞中更喜欢同四聚体构象而不是单体构象; IL28A同源四聚体与HCV NS5A和其他HCV蛋白的自溶酶体降解呈正相关。总之,IL28A蛋白的第一个α螺旋是维持IL28A同四聚体的关键结构域,这是促进自溶酶体形成和体外HCV蛋白降解所必需的。
更新日期:2020-03-24
中文翻译:

IL28A 蛋白同四聚体结构是 HCV-NS5A 体外自溶酶体降解所必需的。
干扰素 lambda-2 (IL28A) 具有广泛的抗病毒作用,且副作用较少。自噬是宿主维持细胞内稳态、防御病原微生物入侵的机制。 HCV NS5A 可以禁用主机防御系统以支持 HCV 复制。因此,本研究关注并探讨干扰素λ、自噬和HCV之间相互作用的分子机制。我们报道HCV NS5A通过促进自噬泛素化样酶ATG3、ATG5、ATG7、ATG10和自噬体标记物LC3B激活不完全自噬,但阻断自噬通量; IL28A 在 NS5A-ISDR 区域与 NS5A 结合,并通过促进 HepG2 细胞中自溶酶体的形成来降解 HCV-NS5A。 IL28A 蛋白构象的软件预测表明了 IL28A 同四聚体的潜在结构; IL28A的第一个α螺旋位于四个IL28A链之间的界面以维持IL28A同源四聚体构象。使用连续缺失突变体的 Co-IP 和细胞免疫荧光实验表明,IL28A 在细胞中更喜欢同四聚体构象而不是单体构象; IL28A同源四聚体与HCV NS5A和其他HCV蛋白的自溶酶体降解呈正相关。总之,IL28A蛋白的第一个α螺旋是维持IL28A同四聚体的关键结构域,这是促进自溶酶体形成和体外HCV蛋白降解所必需的。











































 京公网安备 11010802027423号
京公网安备 11010802027423号