Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
Hypoxia-induced release, nuclear translocation, and signaling activity of a DLK1 intracellular fragment in glioma.
Oncogene ( IF 6.9 ) Pub Date : 2020-03-24 , DOI: 10.1038/s41388-020-1273-9 Elisa Stellaria Grassi 1, 2 , Vasiliki Pantazopoulou 1 , Alexander Pietras 1
Oncogene ( IF 6.9 ) Pub Date : 2020-03-24 , DOI: 10.1038/s41388-020-1273-9 Elisa Stellaria Grassi 1, 2 , Vasiliki Pantazopoulou 1 , Alexander Pietras 1
Affiliation
|
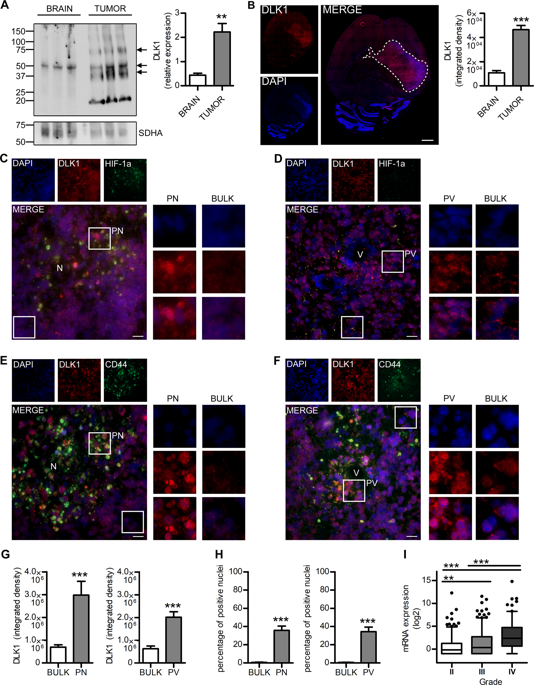
Glioblastoma multiforme is characterized in part by severe hypoxia associated with tumor necrosis. The cellular response to hypoxia can influence several properties of tumor cells associated with aggressive tumor growth, including metabolic adaptations and tumor cell migration and invasion. Here, we found that Delta Like Non-Canonical Notch Ligand 1 (DLK1) expression was elevated as compared with normal brain in a genetically engineered mouse model of glioma, and that DLK1 expression increased with tumor grade in human glioma samples. DLK1 expression was highest in hypoxic and perivascular tumor areas, and we found that hypoxia induced the release and nuclear translocation of an intracellular fragment of DLK1 in murine glioma as well as in human glioma cultures. Release of the intracellular fragment was dependent on ADAM17 and Hypoxia-inducible Factor 1alpha and 2alpha (HIF-1alpha/HIF-2alpha), as ADAM17 inhibitors and HIF1A/HIF2A siRNA blocked DLK1 cleavage. Expression of a cleavable form of DLK1 amplified several hypoxia-induced traits of glioma cells such as colony formation, stem cell marker gene expression, a PI3K-pathway-mediated metabolic shift, and enhanced invasiveness. Effects of DLK1 were dependent on DLK1-cleavage by ADAM17, as expression of non-cleavable DLK1 could not replicate the DLK1-induced hypoxic phenotype. Finally, forced expression of DLK1 resulted in more invasive tumor growth in a PDGFB-induced glioma mouse model without affecting overall survival. Together, our findings suggest a previously undescribed role for DLK1 as an intracellular signaling molecule.
中文翻译:

神经胶质瘤中缺氧诱导的 DLK1 细胞内片段的释放、核转位和信号活动。
多形性胶质母细胞瘤的部分特征是与肿瘤坏死相关的严重缺氧。细胞对缺氧的反应可以影响与侵袭性肿瘤生长相关的肿瘤细胞的多种特性,包括代谢适应以及肿瘤细胞迁移和侵袭。在这里,我们发现,在基因工程胶质瘤小鼠模型中,与正常大脑相比,Delta Like Non-Canonical Notch Ligand 1 (DLK1) 的表达升高,并且在人类胶质瘤样本中,DLK1 的表达随着肿瘤分级的增加而增加。 DLK1 表达在缺氧和血管周围肿瘤区域最高,我们发现缺氧诱导小鼠神经胶质瘤和人类神经胶质瘤培养物中 DLK1 细胞内片段的释放和核转位。细胞内片段的释放依赖于 ADAM17 和缺氧诱导因子 1α 和 2α (HIF-1α/HIF-2α),因为 ADAM17 抑制剂和 HIF1A/HIF2A siRNA 会阻断 DLK1 裂解。 DLK1 可裂解形式的表达放大了神经胶质瘤细胞的几种缺氧诱导的特征,例如集落形成、干细胞标记基因表达、PI3K 途径介导的代谢转变和增强的侵袭性。 DLK1 的作用取决于 ADAM17 对 DLK1 的裂解,因为不可裂解的 DLK1 的表达无法复制 DLK1 诱导的缺氧表型。最后,DLK1 的强制表达导致 PDGFB 诱导的神经胶质瘤小鼠模型中更具侵袭性的肿瘤生长,而不影响总体生存。总之,我们的研究结果表明 DLK1 作为细胞内信号分子具有先前未描述的作用。
更新日期:2020-03-24
中文翻译:

神经胶质瘤中缺氧诱导的 DLK1 细胞内片段的释放、核转位和信号活动。
多形性胶质母细胞瘤的部分特征是与肿瘤坏死相关的严重缺氧。细胞对缺氧的反应可以影响与侵袭性肿瘤生长相关的肿瘤细胞的多种特性,包括代谢适应以及肿瘤细胞迁移和侵袭。在这里,我们发现,在基因工程胶质瘤小鼠模型中,与正常大脑相比,Delta Like Non-Canonical Notch Ligand 1 (DLK1) 的表达升高,并且在人类胶质瘤样本中,DLK1 的表达随着肿瘤分级的增加而增加。 DLK1 表达在缺氧和血管周围肿瘤区域最高,我们发现缺氧诱导小鼠神经胶质瘤和人类神经胶质瘤培养物中 DLK1 细胞内片段的释放和核转位。细胞内片段的释放依赖于 ADAM17 和缺氧诱导因子 1α 和 2α (HIF-1α/HIF-2α),因为 ADAM17 抑制剂和 HIF1A/HIF2A siRNA 会阻断 DLK1 裂解。 DLK1 可裂解形式的表达放大了神经胶质瘤细胞的几种缺氧诱导的特征,例如集落形成、干细胞标记基因表达、PI3K 途径介导的代谢转变和增强的侵袭性。 DLK1 的作用取决于 ADAM17 对 DLK1 的裂解,因为不可裂解的 DLK1 的表达无法复制 DLK1 诱导的缺氧表型。最后,DLK1 的强制表达导致 PDGFB 诱导的神经胶质瘤小鼠模型中更具侵袭性的肿瘤生长,而不影响总体生存。总之,我们的研究结果表明 DLK1 作为细胞内信号分子具有先前未描述的作用。











































 京公网安备 11010802027423号
京公网安备 11010802027423号