Nature Catalysis ( IF 42.8 ) Pub Date : 2020-03-23 , DOI: 10.1038/s41929-020-0434-0 Huan-Ming Huang , Maximilian Koy , Eloisa Serrano , Philipp Miro Pflüger , J. Luca Schwarz , Frank Glorius
|
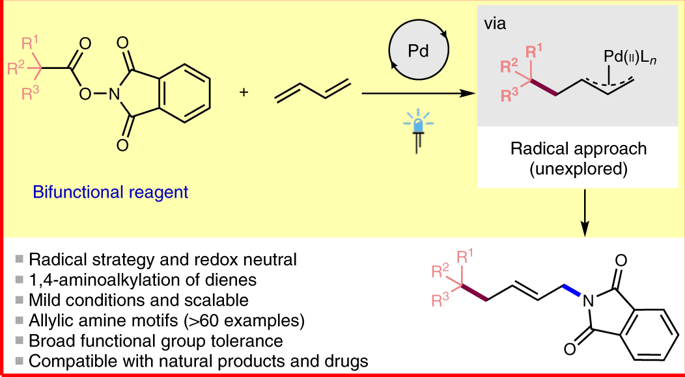
Transition metal catalysed allylic substitution is one of the most powerful and frequently used methods in organic synthesis. In particular, palladium-catalysed allylic functionalization has become a well-established strategy for constructing carbon–carbon or carbon–heteroatom bonds, and its utility has been demonstrated in natural product synthesis, drug discovery and materials science. Several methods have been developed to generate π-allylpalladium complexes through ionic mechanisms; however, these methods typically require either prefunctionalized starting materials or stoichiometric oxidants, which naturally limits their scope. Here, we show a radical approach for the generation of π-allylpalladium complexes by employing N-hydroxyphthalimide esters as bifunctional reagents in combination with 1,3-dienes. Using this strategy, we report the 1,4-aminoalkylation of dienes. The remarkable scope and functional group tolerance of this redox-neutral and mild protocol was demonstrated across >60 examples. The utility of this strategy was further demonstrated in radical cascade reactions and in the late-stage modification of drugs and natural products.

中文翻译:

π-烯丙基铝配合物的催化自由基生成
过渡金属催化的烯丙基取代是有机合成中最有效和最常用的方法之一。特别是,钯催化的烯丙基官能化已成为构建碳-碳或碳-杂原子键的公认策略,其效用已在天然产物合成,药物发现和材料科学中得到证明。已经开发出几种通过离子机理产生π-烯丙基铝配合物的方法。然而,这些方法通常需要预官能化的起始原料或化学计量的氧化剂,这自然限制了它们的范围。在这里,我们展示了一种通过使用N生成π-烯丙基铝配合物的基本方法-羟基邻苯二甲酰亚胺酯作为双功能试剂与1,3-二烯结合使用。使用这种策略,我们报告了二烯的1,4-氨基烷基化。在超过60个示例中证明了此氧化还原中性和温和方案的显着范围和功能组耐受性。自由基级联反应以及药物和天然产物的后期修饰进一步证明了该策略的实用性。












































 京公网安备 11010802027423号
京公网安备 11010802027423号