Nature Medicine ( IF 82.9 ) Pub Date : 2020-03-23 , DOI: 10.1038/s41591-020-0799-2 Samuel W Kazer 1, 2, 3, 4 , Toby P Aicher 1, 2, 4 , Daniel M Muema 5, 6 , Shaina L Carroll 7 , Jose Ordovas-Montanes 1, 2, 3, 4, 8, 9 , Vincent N Miao 1, 2, 4, 10 , Ang A Tu 4, 11, 12 , Carly G K Ziegler 1, 2, 4, 10 , Sarah K Nyquist 1, 2, 4, 13, 14 , Emily B Wong 5, 15, 16, 17 , Nasreen Ismail 6 , Mary Dong 1 , Amber Moodley 6 , Bonnie Berger 14, 18 , J Christopher Love 1, 11, 12 , Krista L Dong 1 , Alasdair Leslie 5, 16 , Zaza M Ndhlovu 1, 5, 6, 19 , Thumbi Ndung'u 1, 5, 6, 16, 20 , Bruce D Walker 1, 6, 19 , Alex K Shalek 1, 2, 3, 4, 10, 11, 13
|
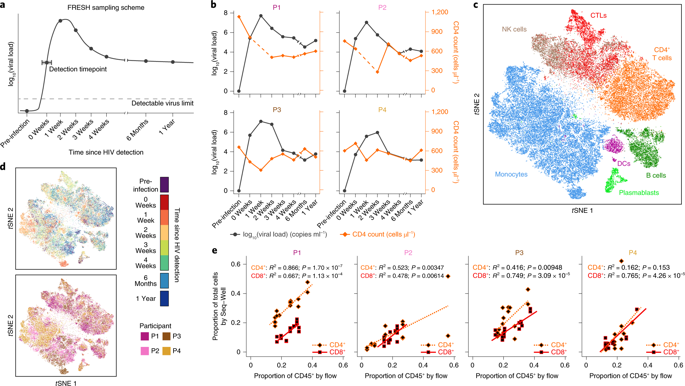
Cellular immunity is critical for controlling intracellular pathogens, but individual cellular dynamics and cell–cell cooperativity in evolving human immune responses remain poorly understood. Single-cell RNA-sequencing (scRNA-seq) represents a powerful tool for dissecting complex multicellular behaviors in health and disease1,2 and nominating testable therapeutic targets3. Its application to longitudinal samples could afford an opportunity to uncover cellular factors associated with the evolution of disease progression without potentially confounding inter-individual variability4. Here, we present an experimental and computational methodology that uses scRNA-seq to characterize dynamic cellular programs and their molecular drivers, and apply it to HIV infection. By performing scRNA-seq on peripheral blood mononuclear cells from four untreated individuals before and longitudinally during acute infection5, we were powered within each to discover gene response modules that vary by time and cell subset. Beyond previously unappreciated individual- and cell-type-specific interferon-stimulated gene upregulation, we describe temporally aligned gene expression responses obscured in bulk analyses, including those involved in proinflammatory T cell differentiation, prolonged monocyte major histocompatibility complex II upregulation and persistent natural killer (NK) cell cytolytic killing. We further identify response features arising in the first weeks of infection, for example proliferating natural killer cells, which potentially may associate with future viral control. Overall, our approach provides a unified framework for characterizing multiple dynamic cellular responses and their coordination.
中文翻译:

超急性 HIV-1 感染期间多细胞免疫动力学的综合单细胞分析
细胞免疫对于控制细胞内病原体至关重要,但人们对进化中的人类免疫反应中的个体细胞动力学和细胞-细胞协同作用仍然知之甚少。单细胞 RNA 测序 (scRNA-seq) 是剖析健康和疾病1,2中复杂的多细胞行为并提名可测试的治疗靶点3的有力工具。将其应用于纵向样本可以提供一个机会来揭示与疾病进展演变相关的细胞因子,而不会潜在地混淆个体间的变异性4. 在这里,我们提出了一种实验和计算方法,该方法使用 scRNA-seq 来表征动态细胞程序及其分子驱动程序,并将其应用于 HIV 感染。通过在急性感染之前和期间纵向对来自四个未经治疗的个体的外周血单核细胞进行 scRNA-seq 5,我们在每个内部都有动力去发现随时间和细胞亚群而变化的基因反应模块。除了以前未被发现的个体和细胞类型特异性干扰素刺激的基因上调外,我们还描述了在批量分析中模糊的时间对齐基因表达反应,包括那些参与促炎 T 细胞分化、延长单核细胞主要组织相容性复合物 II 上调和持续自然杀伤的反应。 NK) 细胞溶解杀伤。我们进一步确定了感染最初几周出现的反应特征,例如增殖的自然杀伤细胞,这可能与未来的病毒控制有关。总体而言,我们的方法为表征多种动态细胞反应及其协调提供了一个统一的框架。



























 京公网安备 11010802027423号
京公网安备 11010802027423号