Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
The Use of α-Diazo-γ-butyrolactams in the Büchner–Curtius–Schlotterbeck Reaction of Cyclic Ketones Opens New Entry to Spirocyclic Pyrrolidones
Synlett ( IF 1.7 ) Pub Date : 2020-03-23 , DOI: 10.1055/s-0040-1708011 Mikhail Krasavin 1 , Maria Eremeyeva , Daniil Zhukovsky , Dmitry Dar’in
Synlett ( IF 1.7 ) Pub Date : 2020-03-23 , DOI: 10.1055/s-0040-1708011 Mikhail Krasavin 1 , Maria Eremeyeva , Daniil Zhukovsky , Dmitry Dar’in
Affiliation
|
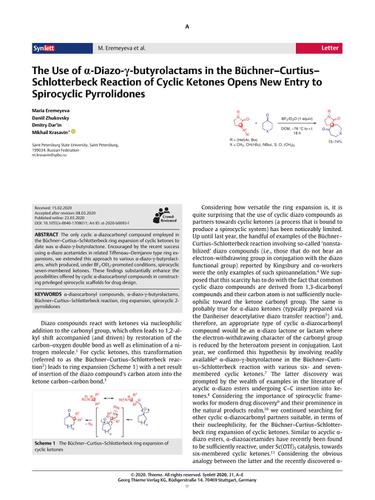
The only cyclic α-diazocarbonyl compound employed in the Buchner–Curtius–Schlotterbeck ring expansion of cyclic ketones to date was α-diazo-γ-butyrolactone. Encouraged by the recent success using α-diazo acetamides in related Tiffeneau–Demjanov type ring expansions, we extended this approach to various α-diazo-γ-butyrolactams, which produced, under BF3·OEt2-promoted conditions, spirocyclic seven-membered ketones. These findings substantially enhance the possibilities offered by cyclic α-diazocarbonyl compounds in constructing privileged spirocyclic scaffolds for drug design.
中文翻译:

α-重氮-γ-丁内酰胺在环酮的 Büchner-Curtius-Schlotterbeck 反应中的应用开辟了螺环吡咯烷酮的新入口
迄今为止,唯一用于环酮的 Buchner-Curtius-Schlotterbeck 扩环的环状 α-重氮羰基化合物是 α-重氮-γ-丁内酯。受到最近在相关 Tiffeneau-Demjanov 型环扩展中使用 α-重氮乙酰胺的成功鼓舞,我们将这种方法扩展到各种 α-重氮-γ-丁内酰胺,在 BF3·OEt2 促进的条件下产生螺环七元酮。这些发现大大增强了环状 α-重氮羰基化合物在构建用于药物设计的特殊螺环支架方面的可能性。
更新日期:2020-03-23
中文翻译:

α-重氮-γ-丁内酰胺在环酮的 Büchner-Curtius-Schlotterbeck 反应中的应用开辟了螺环吡咯烷酮的新入口
迄今为止,唯一用于环酮的 Buchner-Curtius-Schlotterbeck 扩环的环状 α-重氮羰基化合物是 α-重氮-γ-丁内酯。受到最近在相关 Tiffeneau-Demjanov 型环扩展中使用 α-重氮乙酰胺的成功鼓舞,我们将这种方法扩展到各种 α-重氮-γ-丁内酰胺,在 BF3·OEt2 促进的条件下产生螺环七元酮。这些发现大大增强了环状 α-重氮羰基化合物在构建用于药物设计的特殊螺环支架方面的可能性。











































 京公网安备 11010802027423号
京公网安备 11010802027423号