当前位置:
X-MOL 学术
›
J. Alloys Compd.
›
论文详情
Our official English website, www.x-mol.net, welcomes your
feedback! (Note: you will need to create a separate account there.)
First principles investigation on interface properties and formation mechanism of γ-Fe/CeO2 heterogeneous nucleation interface
Journal of Alloys and Compounds ( IF 5.8 ) Pub Date : 2020-08-01 , DOI: 10.1016/j.jallcom.2020.154867 Xiaoyong Jiao , Wantang Fu , Zhijun Shi , Zhengjun Li , Yefei Zhou , Xiaolei Xing , Zirong Wang , Qingxiang Yang
Journal of Alloys and Compounds ( IF 5.8 ) Pub Date : 2020-08-01 , DOI: 10.1016/j.jallcom.2020.154867 Xiaoyong Jiao , Wantang Fu , Zhijun Shi , Zhengjun Li , Yefei Zhou , Xiaolei Xing , Zirong Wang , Qingxiang Yang
|
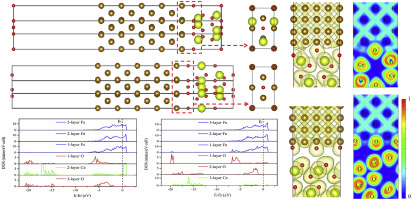
Abstract The first principles method was used to calculate the interface stability, interfacial electronic structure and bond characteristics of γ-Fe/CeO2 heterogeneous nucleation interface. Meanwhile, The formation mechanism of (100)_γ-Fe/(111)_CeO2 heterogeneous nucleation interface was also revealed. The calculation results show that the two dimensional lattice mismatch between (100)_γ-Fe and (111)_CeO2 is 4.9%, which indicates that (100)_γ-Fe and (111)_CeO2 satisfy the crystallographic condition for the formation of effective heterogeneous nucleation interface. There are two kinds of interface models, named as O1-Fe and O2-Fe. The stability of O1-Fe interface is the best and its interfacial energy is the lowest, which is 3.07 J/m2. The ideal interfacial adhesive strength of O2-Fe interface is the largest, which is 10.98 J/m2. The bond characteristics of the two interfaces are both mixtures of covalent bonds and ionic bonds. It reveals that (100)_γ-Fe and (111)_CeO2 can form effective heterogeneous nucleation interfaces and O1-Fe interface model forms preferably during the heterogeneous nucleation beginning process. When the formation conditions meet the requirement for both interface structures, the adhesive strength of the O2-Fe interface is higher. Therefore, CeO2 can act as effective heterogeneous nucleation substrate of γ-Fe to refine the austenite grains.
中文翻译:

γ-Fe/CeO2异相形核界面界面性质及形成机制的第一性原理研究
摘要 采用第一性原理计算γ-Fe/CeO2异相成核界面的界面稳定性、界面电子结构和键合特性。同时,还揭示了(100)_γ-Fe/(111)_CeO2异相形核界面的形成机制。计算结果表明,(100)_γ-Fe和(111)_CeO2的二维晶格失配为4.9%,表明(100)_γ-Fe和(111)_CeO2满足形成有效异质的结晶条件。成核界面。有两种界面模型,分别称为 O1-Fe 和 O2-Fe。O1-Fe界面的稳定性最好,界面能最低,为3.07 J/m2。O2-Fe界面的理想界面粘合强度最大,为10.98 J/m2。两个界面的键合特性都是共价键和离子键的混合体。结果表明,(100)_γ-Fe和(111)_CeO2可以形成有效的异相形核界面,并且在异相形核开始过程中最好形成O1-Fe界面模型。当形成条件满足两种界面结构的要求时,O2-Fe界面的粘合强度更高。因此,CeO2 可以作为γ-Fe 的有效异质形核基体来细化奥氏体晶粒。当形成条件满足两种界面结构的要求时,O2-Fe界面的粘合强度更高。因此,CeO2 可以作为γ-Fe 的有效异质形核基体来细化奥氏体晶粒。当形成条件满足两种界面结构的要求时,O2-Fe界面的粘合强度更高。因此,CeO2 可以作为γ-Fe 的有效异质形核基体来细化奥氏体晶粒。
更新日期:2020-08-01
中文翻译:

γ-Fe/CeO2异相形核界面界面性质及形成机制的第一性原理研究
摘要 采用第一性原理计算γ-Fe/CeO2异相成核界面的界面稳定性、界面电子结构和键合特性。同时,还揭示了(100)_γ-Fe/(111)_CeO2异相形核界面的形成机制。计算结果表明,(100)_γ-Fe和(111)_CeO2的二维晶格失配为4.9%,表明(100)_γ-Fe和(111)_CeO2满足形成有效异质的结晶条件。成核界面。有两种界面模型,分别称为 O1-Fe 和 O2-Fe。O1-Fe界面的稳定性最好,界面能最低,为3.07 J/m2。O2-Fe界面的理想界面粘合强度最大,为10.98 J/m2。两个界面的键合特性都是共价键和离子键的混合体。结果表明,(100)_γ-Fe和(111)_CeO2可以形成有效的异相形核界面,并且在异相形核开始过程中最好形成O1-Fe界面模型。当形成条件满足两种界面结构的要求时,O2-Fe界面的粘合强度更高。因此,CeO2 可以作为γ-Fe 的有效异质形核基体来细化奥氏体晶粒。当形成条件满足两种界面结构的要求时,O2-Fe界面的粘合强度更高。因此,CeO2 可以作为γ-Fe 的有效异质形核基体来细化奥氏体晶粒。当形成条件满足两种界面结构的要求时,O2-Fe界面的粘合强度更高。因此,CeO2 可以作为γ-Fe 的有效异质形核基体来细化奥氏体晶粒。











































 京公网安备 11010802027423号
京公网安备 11010802027423号